July 19, 2016

Device Simplifies Valve Repair, Avoids Open Heart Surgery
Researchers investigating a novel device to repair the mitral heart valve report 100 percent procedural success in a safety and performance study, the first such study done in humans. The image-guided device, based on technology developed at the University of Maryland School of Medicine, is deployed through a tiny opening in a beating heart, avoids open-heart surgery, automates a key part of the valve repair process, simplifies the procedure and reduces operating room time. The research is published in the journal Circulation.
Traditional mitral valve repair is performed during open heart surgery, a lengthy operation in which the patient’s chest is opened, the heart is stopped and circulation is maintained with a heart-lung bypass machine. Recovery can take months, and patients face significant risks. As a result, there is considerable interest in finding less invasive mitral valve treatment options.
“We think this is a safer approach than open heart surgery,” says principal investigator James S. Gammie, MD, professor and chief of cardiac surgery at the University of Maryland School of Medicine. “We think the safety profile is going to be better and, ultimately, people will be able to go home from the hospital the next day.”
The Device
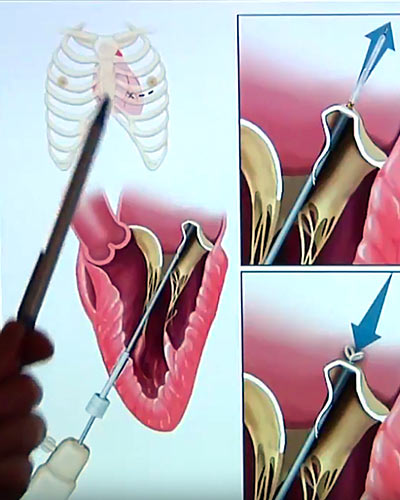
The device, known as the Harpoon TSD-5, made by Harpoon Medical Inc. of Baltimore, is an investigational device. At the present time, the US Food and Drug Administration has not approved the device for use in patients in the United States. It is designed to treat degenerative mitral regurgitation (MR), the most common type of heart valve disorder. In MR, a leaky valve lets blood travel in the wrong direction on the left side of the heart, causing shortness of breath, fluid retention, irregular heartbeats and fatigue. MR develops when the small fibrous cords that open and close the valve’s flaps, known as leaflets, are broken or stretched, preventing them from closing tightly and causing the leaflets to bulge or prolapse upward toward the left atrium. The natural cords connect the valve flaps to muscles inside the heart that contract to close the mitral valve, which gets its name because its two flaps resemble a bishop’s mitre.
The TSD-5 anchors artificial cords on the flaps to take the place of the natural cords. The artificial cords are made of expanded polytetrafluoroethylene (ePTFE), a polymer commonly used as sutures in cardiac surgery.
Surgeons insert the device into the beating heart through a tiny opening in the ribcage and, using echocardiographic imaging, guide it to the surface of the defective mitral flaps. When the surgeon determines the optimal placement for an artificial cord, the device is actuated and a specially designed needle wrapped with 50 coils of ePTFE makes a tiny hole and sends the cord material through the flap. An automated process makes a knot to hold the cord in place. The other end of the cord is adjusted for optimum length and tied to the outside layer of the heart, the epicardium. Three or four cords are required for most cases.
Gammie says the ability to make adjustments to the artificial cords while the heart is beating is a key advantage over open heart surgery: “The heart’s fully loaded and beating and we can just adjust the length of the cords to optimize the result, and only when we’re really happy do we tie it off.”
Study Design
Patients with severe degenerative MR resulting from isolated posterior leaflet prolapse were enrolled. Patients were selected if there was adequate leaflet surface to result in effective MR reduction. The primary outcome was procedural success, defined as successful implantation of one or more ePTFE artificial cords on the mitral valve, as well as a reduction in MR from severe to less than or equal to moderate at the conclusion of the procedure and at 30 days.
The study focused on an initial 11 patients at two clinical centers in Poland. Surgeons used the device to successfully implant multiple artificial cords and effectively reduce MR in all patients. The investigators say results were stable at 30 days and the procedure was safe.
Mitral valve regurgitation affects an estimated two percent of the population in the United States, or nearly eight million people. “We think this approach is applicable to probably three-quarters of patients with degenerative disease, which is the number one reason why people come to the operating room for surgery for mitral valve regurgitation,” says Gammie.
“Mitral valve surgery requires great skill and experience to produce good patient outcomes, expertise that our cardiac surgery faculty demonstrate on a daily basis,” says E. Albert Reece, MD, PhD, MBA, vice president for medical affairs at the University of Maryland and the John Z. and Akiko K. Bowers Distinguished Professor and dean of the University of Maryland School of Medicine. “We are proud to see that a device that began as an idea in the laboratories of the University of Maryland School of Medicine may help improve outcomes for even more patients.”
DISCLOSURE: The study was funded by Harpoon Medical, Inc. Dr. Gammie and three others on the research team report ownership of stock and/or options to purchase stock in Harpoon Medical. Another member of the study team, Mehrdad Ghoreishi, MD, is a co-inventor of the technology, which has been licensed from the University of Maryland by Harpoon Medical. At the present time, the US Food and Drug Administration has not approved the Harpoon TSD-5 for patients in the United States.
###
Gammie JS, Wilson P, Bartus K, Gackowski A, Hung J, D’Ambra MN, Kolsut P, Bittle GJ, Szymanski P, Sadowski J, Kapelak B, Bilewska A, Kusmierczyk M, Ghoreishi M. “Transapical beating-heart mitral valve repair with an ePTFE cordal implantation device: Initial clinical experience.” Circulation. Published online ahead of print, July 19, 2016. http://dx.doi.org/10.1161/CIRCULATIONAHA.116.022010
About the University of Maryland School of Medicine
The University of Maryland School of Medicine was chartered in 1807 and is the first public medical school in the United States. It continues today as an innovative leader in accelerating innovation and discovery in medicine. The School of Medicine is the founding school of the University of Maryland and is an integral part of the 11-campus University System of Maryland. Located on the University of Maryland’s Baltimore campus, the School of Medicine works closely with the University of Maryland Medical Center and Medical System to provide a research-intensive, academic and clinically based education. With 43 academic departments, centers and institutes and a faculty of more than 3,000 physicians and research scientists plus more than $400 million in extramural funding, the School is regarded as one of the leading biomedical research institutions in the U.S. with top-tier faculty and programs in cancer, brain science, surgery and transplantation, trauma and emergency medicine, vaccine development and human genomics, among other centers of excellence. The School is not only concerned with the health of the citizens of Maryland and the nation, but also has a global presence, with research and treatment facilities in more than 35 countries around the world. http://medschool.umaryland.edu.
About the University of Maryland Medical Center
The University of Maryland Medical Center (UMMC) is comprised of two hospitals in Baltimore: an 800-bed teaching hospital — the flagship institution of the 12-hospital University of Maryland Medical System (UMMS) — and a 200-bed community teaching hospital, UMMC Midtown Campus. UMMC is a national and regional referral center for trauma, cancer care, neurocare, cardiac care, diabetes and endocrinology, women's and children's health, and has one of the largest solid organ transplant programs in the country. All physicians on staff at the flagship hospital are faculty physicians of the University of Maryland School of Medicine. At UMMC Midtown Campus, faculty physicians work alongside community physicians to provide patients with the highest quality care. UMMC Midtown Campus was founded in 1881 and is located one mile away from the University Campus hospital. For more information, visit www.umm.edu.
Contact
Department of Anesthesiology
(410) 328-6120 (phone)
(410) 328-5531 (fax)
swalsh@som.umaryland.edu
Bill Seiler
Media Relations
410-328-8919
bseiler@umm.edu
Related stories

Friday, July 20, 2018
Delaying Mitral Valve Surgery May Harm Patients
Study Shows Earlier Surgery Gets Better Results
Friday, December 01, 2017
New Study: Innovative Heart Device is Safe and Effective
A new study has found that a pioneering device to repair heart valves is safe and effective, and can reduce the invasiveness and side effects of conventional mitral valve surgery. The Harpoon Mitral Valve Repair System (H-MVRS), an image-guided device based on technology developed at the University of Maryland School of Medicine (UMSOM), is deployed through a small opening between the ribs, and repairs the heart while it continues to beat. The research was presented at the Transcatheter Cardiovascular Therapeutics (TCT 2017) symposium in Denver, and simultaneously published in JACC, the Journal of the American College of Cardiology.

Thursday, March 10, 2016
UM SOM Establishes Two Endowed Professorships Through Private Gifts and Matching State Funds
University of Maryland School of Medicine (UM SOM) Dean E. Albert Reece, MD, PhD, MBA, announced today that the School has been awarded matching funds from the Maryland Department of Business and Economic Development (DBED) as part of the Maryland E-Nnovation Initiative Fund program. The funds, when combined with private philanthropy, will enable UM SOM to establish two new endowed professorships – one in human virology and vaccine development, the other in surgical science and entrepreneurship.
