December 18, 2023 | Lauren Wright

The mission of the Confocal Microscopy Core at the University of Maryland School of Medicine’s (UMSOM) Center for Innovative Biomedical Resources (CIBR) is to provide researchers with a wide array of state-of-the-art microscopy equipment, including one of its latest technologies, the Zeiss LS7 Lightsheet microscope. Heavily invested in providing hardware and software to facilitate high-impact data collection and analysis, the School of Medicine acquired the LS7 Lightsheet microscope in 2022 after receiving $600,000 from the National Institutes of Health (NIH) and over $110,000 in funding from departments including Pharmacology, Neurobiology, Neurosurgery, and Physiology.
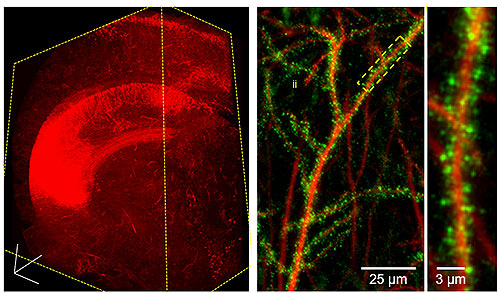 Lightsheet fluorescence microscopy is an important new method for imaging intact organoids, organs, tissues, and organisms, both in fixed, cleared samples or in live specimens and embryos. This imaging modality fills an important gap in the technical arsenal of the university, which has excellent facilities for imaging at scales from electron microscopy to PET/CT but lacked a microscope optimized for high-resolution, large-volume imaging.
Lightsheet fluorescence microscopy is an important new method for imaging intact organoids, organs, tissues, and organisms, both in fixed, cleared samples or in live specimens and embryos. This imaging modality fills an important gap in the technical arsenal of the university, which has excellent facilities for imaging at scales from electron microscopy to PET/CT but lacked a microscope optimized for high-resolution, large-volume imaging.
The LS7 now allows researchers to visualize biological specimens rapidly and with high spatial resolution in intact, clear tissues and organs. In combination with advanced tissue clearing methods, lightsheet microscopy permits dramatic 3D and multicolor visualization of organ structure, cellular architecture, and subcellular constituents. Currently, researchers at the University of Maryland, Baltimore (UMB) have visualized brain, lung, bone, and zebrafish. Time-lapse imaging of fluorescent biosensors in living zebrafish has also been achieved, demonstrating the wide range of applications for this microscope.
 The new capabilities of the LS7 Lightsheet microscope have allowed researchers unprecedented access to biological data in multiple organ systems. In neurobiology, imaging of cleared brains has shown the location of proteins in neuronal spines, the distribution of specific cell types in the maturing brain, and neuronal circuitry and projections. Additionally, the intricate branching of the cerebrovascular network has been visualized, thus opening new avenues for investigation. Research in pulmonary physiology has determined the distribution of pharmacological agents in the pulmonary airways. The new microscope therefore continues to facilitate new discoveries for researchers at UMB.
The new capabilities of the LS7 Lightsheet microscope have allowed researchers unprecedented access to biological data in multiple organ systems. In neurobiology, imaging of cleared brains has shown the location of proteins in neuronal spines, the distribution of specific cell types in the maturing brain, and neuronal circuitry and projections. Additionally, the intricate branching of the cerebrovascular network has been visualized, thus opening new avenues for investigation. Research in pulmonary physiology has determined the distribution of pharmacological agents in the pulmonary airways. The new microscope therefore continues to facilitate new discoveries for researchers at UMB.
Equipped with six illumination laser lines and two separate sCMOS cameras, the imaging capabilities of the microscope are diverse. The LS7 can image samples in both aqueous and clearing media, and a range of objective lenses and chambers permit imaging a wide array of sample sizes and effective magnifications. Imaging of live specimens such as zebrafish is readily permitted by the unique sample holders in the microscope. Once data is acquired, researchers have the choice of analysis software packages, including Arivis and Imaris Bitplane, which are available to conduct advanced 3D and 4D data analysis.
 “Under Tom Blanpied’s leadership, the School of Medicine is becoming a leading center in imaging the brain and body at the cellular, subcellular and molecular level,” said Margaret McCarthy, PhD, the James & Carolyn Frenkil Dean’s Professor and Chair of the Department of Pharmacology at UMSOM and Director of the University of Maryland - Medicine Institute of Neuroscience Discovery (UM-MIND). “Super-resolution microscopy is revealing unprecedented visualization of neurons, cancer cells, heart muscle and bones that advances our knowledge of healthy tissues and how they are impacted by injury and disease.”
“Under Tom Blanpied’s leadership, the School of Medicine is becoming a leading center in imaging the brain and body at the cellular, subcellular and molecular level,” said Margaret McCarthy, PhD, the James & Carolyn Frenkil Dean’s Professor and Chair of the Department of Pharmacology at UMSOM and Director of the University of Maryland - Medicine Institute of Neuroscience Discovery (UM-MIND). “Super-resolution microscopy is revealing unprecedented visualization of neurons, cancer cells, heart muscle and bones that advances our knowledge of healthy tissues and how they are impacted by injury and disease.”
Available to all UMB researchers and extramural users on a fee-for-service basis, the microscope is currently housed on the 9th floor of the Health Sciences Facility III (HSF III). However, plans are underway to relocate the LS7 to the 6th floor of the building within the newly renovated space of the UM-MIND.
Training and assistance in the use of the microscope is available through the Confocal Microscopy Facility. Researchers will receive instruction in optimization of data acquisition and image processing. Following training, access to the instrument is available via CIBR's online iLab Cores Portal.
For more information on training and how to connect with the entire network of LS7 users, contact Joseph Ryan H. Mauban, PhD, Manager, Confocal Microscopy Core, by email: jmauban@umaryland.edu. Dr. Mauban recommends scientists at UMSOM stay tuned for even more possibilities. Additional imaging technologies will be available in 2024 and announced soon.
Contact
Lauren Wright
Public Affairs/Community Health Specialist
Managing Editor, SOM News
Office of Public Affairs & Communications
University of Maryland School of Medicine
LaurenWright@som.umaryland.edu
Office: 410.706.7508
Work Cell: 443.743.9047
Related stories

Monday, November 06, 2023
UMSOM Faculty Speakers Deliver Inspirational Messages at UMB’s 2nd Annual Faculty Convocation
Two faculty from the University of Maryland School of Medicine (UMSOM), along with Glenn Canares, DDS, MSD, Clinical Assistant Professor at the University of Maryland School of Dentistry, delivered compelling keynote addresses at the UMB Faculty Convocation held on September 14, 2023. With the hope of inspiring faculty and staff at the beginning of the new academic year, the celebration recognized faculty achievements and honored the 2023-2024 cohort of UMB Distinguished University Professors.
