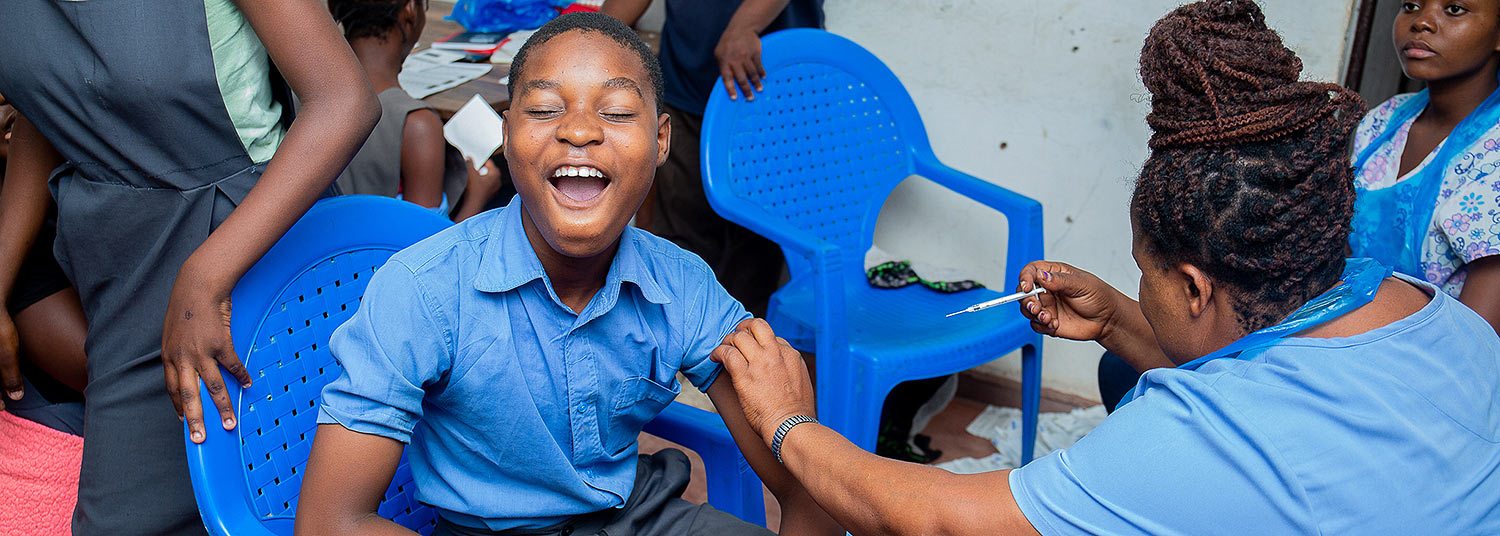January 25, 2024 | Deborah Kotz
First Study in Africa Found TCV Provided More than Four Years of Protection in Those Ages 9 Months & Older
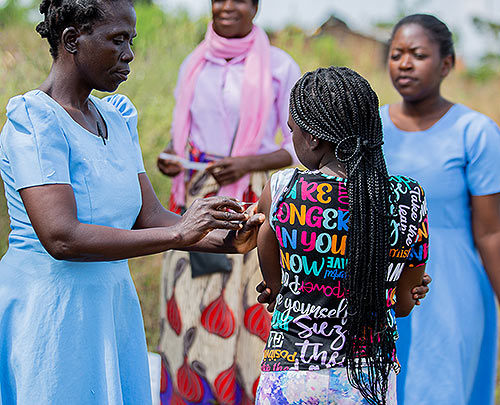 A single dose of the typhoid conjugate vaccine, Typbar TCV®, provides lasting efficacy in preventing typhoid fever in children ages 9 months to 12 years old, according to a new study conducted by researchers at University of Maryland School of Medicine’s (UMSOM) Center for Vaccine Development and Global Health (CVD) and led by in-country partners at the Malawi-Liverpool Wellcome Trust (MLW) Clinical Research Programme.
A single dose of the typhoid conjugate vaccine, Typbar TCV®, provides lasting efficacy in preventing typhoid fever in children ages 9 months to 12 years old, according to a new study conducted by researchers at University of Maryland School of Medicine’s (UMSOM) Center for Vaccine Development and Global Health (CVD) and led by in-country partners at the Malawi-Liverpool Wellcome Trust (MLW) Clinical Research Programme.
Results from the phase 3 clinical study were published today in The Lancet.
The research team enrolled more than 28,000 healthy children in Malawi and randomly assigned about half the group to receive the TCV and the other half to receive a meningococcal capsular group A conjugate (MenA) control vaccine. During the more than four years of follow-up, 24 children in the TCV group and 110 in the MenA group developed typhoid fever, which was confirmed via blood culture. That resulted in an efficacy of 78.3 percent in the TCV group, with one case of typhoid prevented for every 163 children vaccinated. TCV was effective in all age groups and over the study period – which ended in 2022 – vaccine efficacy remained strong, decreasing by only 1.3 percent per year.
“The newly published study supports the long-lasting impacts of a single shot of TCV, even in the youngest children, and offers hope of preventing typhoid in the most vulnerable children,” said Kathleen Neuzil, MD, MPH, CVD Director, the Myron M. Levine, MD, DTPH, Professor in Vaccinology at UMSOM and coauthor of the current study. “We could not have had a better partner in this endeavor than MLW, whose long-standing excellence in typhoid research and strong surveillance infrastructure made this study possible.”
 Typhoid fever causes more than 9 million illnesses and at least 110,000 deaths worldwide every year, mostly in sub-Saharan Africa and southeast Asia. It is a contagious bacterial infection that occurs from consuming contaminated food or beverages. Symptoms include nausea, fever, and abdominal pain that, if left untreated, can be deadly.
Typhoid fever causes more than 9 million illnesses and at least 110,000 deaths worldwide every year, mostly in sub-Saharan Africa and southeast Asia. It is a contagious bacterial infection that occurs from consuming contaminated food or beverages. Symptoms include nausea, fever, and abdominal pain that, if left untreated, can be deadly.
“These findings have significant implications for identification of the contribution of TCVs in the control and potential elimination of typhoid fever in endemic settings,” wrote the authors of a commentary published in The Lancet alongside the study.
In May 2023, the Malawi government launched a national rollout of the TCV in children under 15 years. Going forward, all children in Malawi will receive TCV at nine months of age as part of the routine immunization schedule.
 “The CVD’s outstanding record of generating data to accelerate public health decisions continues with this landmark study,” said UMSOM Dean Mark T. Gladwin, MD, Vice President for Medical Affairs, University of Maryland, Baltimore, and the John Z. and Akiko K. Bowers Distinguished Professor. “The research could not come at a more critical time when Malawi and other African countries are struggling with climate change, extreme weather events and increased urbanization patterns, which are likely to contribute to increases in enteric diseases, including typhoid.”
“The CVD’s outstanding record of generating data to accelerate public health decisions continues with this landmark study,” said UMSOM Dean Mark T. Gladwin, MD, Vice President for Medical Affairs, University of Maryland, Baltimore, and the John Z. and Akiko K. Bowers Distinguished Professor. “The research could not come at a more critical time when Malawi and other African countries are struggling with climate change, extreme weather events and increased urbanization patterns, which are likely to contribute to increases in enteric diseases, including typhoid.”
Resources for the Media:
Please download images and video to your computer before viewing them.
TyVAC is funded by the Bill & Melinda Gates Foundation. Typbar TCV® is licensed by Bharat Biotech International Limited, Hyderabad, India.
 Healthy children in Malawi participating in study to test efficacy of typhoid conjugate vaccine. Credit for all study photos: TyVAC/Madalitso Mvula
Healthy children in Malawi participating in study to test efficacy of typhoid conjugate vaccine. Credit for all study photos: TyVAC/Madalitso Mvula
About the University of Maryland School of Medicine
Now in its third century, the University of Maryland School of Medicine was chartered in 1807 as the first public medical school in the United States. It continues today as one of the fastest growing, top-tier biomedical research enterprises in the world -- with 46 academic departments, centers, institutes, and programs, and a faculty of more than 3,000 physicians, scientists, and allied health professionals, including members of the National Academy of Medicine and the National Academy of Sciences, and a distinguished two-time winner of the Albert E. Lasker Award in Medical Research. With an operating budget of more than $1.2 billion, the School of Medicine works closely in partnership with the University of Maryland Medical Center and Medical System to provide research-intensive, academic, and clinically based care for nearly 2 million patients each year. The School of Medicine has more than $500 million in extramural funding, with most of its academic departments highly ranked among all medical schools in the nation in research funding. As one of the seven professional schools that make up the University of Maryland, Baltimore campus, the School of Medicine has a total population of nearly 9,000 faculty and staff, including 2,500 students, trainees, residents, and fellows. The School of Medicine, which ranks as the 8th highest among public medical schools in research productivity (according to the Association of American Medical Colleges profile) is an innovator in translational medicine, with 606 active patents and 52 start-up companies. In the latest U.S. News & World Report ranking of the Best Medical Schools, published in 2023, the UM School of Medicine is ranked #10 among the 92 public medical schools in the U.S., and in the top 16 percent (#32) of all 192 public and private U.S. medical schools. The School of Medicine works locally, nationally, and globally, with research and treatment facilities in 36 countries around the world. Visit medschool.umaryland.edu
About the Center for Vaccine Development and Global Health at the University of Maryland School of Medicine
For over 40 years, researchers in the Center for Vaccine Development and Global Health (CVD) have worked domestically and internationally to develop, test, and deploy vaccines to aid the world’s underserved populations. CVD is an academic enterprise engaged in the full range of infectious disease intervention from basic laboratory research through vaccine development, pre-clinical and clinical evaluation, large-scale pre-licensure field studies, and post-licensure assessments. CVD has created and tested vaccines against cholera, typhoid fever, paratyphoid fever, non-typhoidal Salmonella disease, shigellosis (bacillary dysentery), Escherichia coli diarrhea, nosocomial pathogens, tularemia, influenza, coronaviruses, malaria, and other infectious diseases. CVD’s research covers the broader goal of improving global health by conducting innovative, leading research in Baltimore and around the world. Our researchers are developing new and improved ways to diagnose, prevent, treat, control, and eliminate diseases of global impact, including COVID-19. In addition, CVD’s work focuses on the ever-growing challenge of antimicrobial resistance.
Contact
Deborah Kotz
Senior Director of Media Relations
Office of Public Affairs & Communications
University of Maryland School of Medicine
Email: DKotz@som.umaryland.edu
o: 410-706-4255
t: @debkotz2