Academic Title:
Professor
Primary Appointment:
Medicine
Secondary Appointment(s):
Medical and Research Technology, Microbiology and Immunology
Administrative Title:
Director of Education for the Center of Vaccine Development & Global Health
Location:
HSF-II, S363
Phone (Primary):
(410) 706-3702
Education and Training
University of Delaware, B.A., Biology, 1985
Virginia Commonwealth University Medical College of Virginia, Ph.D., Microbiology and Immunology, 1991
Postdoctoral Fellowship, University of Maryland School of Medicine Center for Vaccine Development, 1992-1994
Postdoctoral Fellowship, University of Maryland School of Medicine Center for Vaccine Development, 1994-1996
Biosketch
Dr. Barry's research in the Center for Vaccine Development and Global Health focuses on the development of vaccines against enteric pathogens, including Shigella and enterotoxigenic E. coli (ETEC), that are responsible for significant morbidity and mortality throughout the world. Target populations include children in less developed counties who suffer the largest burden as well as travelers to endemic regions and deployed military personnel. These pathogens cause infection following ingestion and our strategy is to develop live attenuated strains that are delivered via the oral route. She has constructed live attenuated vaccine candidates of Shigella that have advanced to Phase I clinical trials where they have been demonstrated to be safe and immunogenic. The attenuated bacteria are also used as live vectors for the expression and delivery of antigens from heterologous pathogens. Dr. Barry has engineered a vaccine composed of attenuated Shigella that expresses critical ETEC antigens. This multivalent vaccine is currently in Phase I clinical studies.
An additional area of research is the development of vaccines against Francisella tularensis, a pathogen of interest for bioterror countermeasure development. Vaccine candidates engineered with specific mutations and attenuation, as well as immunogenicity, are currently being evaluated.
Research/Clinical Keywords
Enteric bacterial pathogens, Shigella, pathogenic E. coli, Francisella tularensis, biodefense, diarrheal disease, intracellular pathogens, bacterial-host interactions, macrophage models, enteroid, in vitro organ culture (IVOC).
Highlighted Publications
Delaine B, Wu T, Grassel C, Shimanovich A, Pasetti M, Levine MM, Barry EM. Characterization of a multicomponent live, attenuated Shigella flexneri vaccine. Pathog Dis. 2016 Jul;74(5).
Santiago, AE, Mann BJ, Qin A, Cunningham AL, Cole LE, Vogel SN, Grassel C, Levine MM, Barry EM. Characterization of Francisella tularensis Schu S4 defined mutants as live vaccine candidates. Pathog Dis. 2015;73:ftv036.
Reed DS, Smith L, Cole KS, Santiago AE, Mann BJ, Barry EM. Live attenuated mutants of Francisella tularensis protect rabbits against aerosol challenge with a virulent type A strain. Infect Immun. 2014 May;82(5):2098-105.
Barry, EM, Pasetti MF, Sztein MB, Kotloff KL, Fasano F, Levine MM. Progress and pitfalls in Shigella vaccine research. Nat Rev Gastroenterol Hepatol. 2013 Apr;10(4):245-55.
Additional Publication Citations
Delaine B, Wu T, Grassel C, Shimanovich A, Pasetti M, Levine MM, Barry EM. Characterization of a multicomponent live, attenuated Shigella flexneri vaccine. Pathog Dis. 2016 July;74(5): pii: ftw034.
Hazen TH, Donnenberg MS, Panchalingam S, Antonio M, Hossain A, Mandomando I, Ochieng JB, Ramamurthy T, Tamboura B, Qureshi S, Quadri F, Zaidi A, Kotloff KL, Levine MM, Barry EM, Kaper JB, Rasko DA, Nataro JP. Genomic diversity of EPEC associated with clinical presentations of differing severity. Nature Microbiology. 2016 Jan; Article number:15014.
Santiago AE, Mann BJ, Qin A, Cunningham AL, Cole LE, Vogel SN, Grassel C, Levine MM, Barry EM. Characterization of Francisella tularensis Schu S4 defined mutants as live vaccine candidates. Pathog Dis. 2015 Aug;73(6):ftv036.
Livio S, Strockbrine N, Panchalingam S, Tennant SM, Barry EM, Marohn ME, Antonio M, Hossain A, Mandomando I, Ochieng JB, Oundo JO, Qureshi S, Ramamurthy T, Tamboura B, Adegbola RA, Hossain MJ, Saha D, Sen S, Syed A, Faruque G, Alonso PL, Breiman RF, Zaidi AKM, Sur D, Sow SO, Berkeley LY, Mintz ED, Biwas K, Cohen D, Farag T, Nasrin D, Kotloff KL, Nataro JP, Levine MM. Shigella isolates from the Global Enteric Multicenter Study (GEMS) inform vaccine development. Clin Infect Dis. 2014 Oct 1;59(7):933-941.
Morris CR, Grassel CL, Redman JC, Sahl JW, Barry EM*, Rasko DA*. Characterization of intracellular growth regulator icgR by utilizing transcriptomics to identify mediators of pathogenesis in Shigella flexneri. Infect Immun. 2013 Sep;81(9):3068-3076. *authors contributed equally to work.
Marohn ME and Barry EM. Live attenuated tularemia vaccines: Recent developments and future goals. Vaccine. 2013;31:3485-91.
Barry EM, Pasetti MF, Sztein MB, Kotloff KL, Fasano A, Levine MM. Progress and pitfalls in Shigella vaccine research. Nat Rev Gastroenterol Hepatol. 2013 Apr;10(4):245-255.
Faherty CS, Harper JM, Shea-Donahue T, Barry EM, Kaper JB, Fasano A, Nataro JP. Chromosomal and plasmid-encoded factors of Shigella flexneri induce secretogenic activity ex vivo. PLoS One. 2012;7(11):e49980.
Faherty CS, Redman JC, Rasko DA, Barry EM*, Nataro JP*. Shigella flexneri effectors OspE1 and OspE2 mediate induced adherence to the colonic epithelium following bile salts exposure. Molec. Microbiol. 2012;85(1):107-121. *authors contributed equally to work.
Marohn, ME, Santiago AE, Shirey KA, Lipsky M, Vogel SN, Barry EM. Members of the Francisella tularensis phagosomal transporter subfamily of major facilitator superfamily transporters are critical for pathogenesis. Infection & Immun. 2012 Jul;80(7):2390-2401.
Wu T, Grassel C, Levine MM, Barry EM. Live attenuated Shigella dysenteriae 1 vaccine strains overexpressing Shiga toxin B subunit. Infection & Immun. 2011;79(12):4912-4922.
Reed DS, Smith L, Dunsmore T, Trichel A, Ortiz LA, Cole KS, Barry EM. Pneumonic tularemia in rabbits resembles the human disease as illustrated by radiogrphic and hematological changes after infection. PLoS One. 2011;6(9):e24654
Ohtake S, Martin RA, Saxena A, Lechuga-Ballesteros D, Santiago AE, Barry EM, Truong-Le V. Formulation and stabilization of Francisella tularensis live vaccine strain. J Pharm Sci. 2011;100:3076-3087.
Barry EM, Cole L, Santiago A. Vaccines against tularemia. Human Vaccines. 2009 Dec;5(12):832-838.
Cole LE, Laird MHW, Seekatz A, Santiago A, Jiang Z, Barry EM, Shirey KA, Fitzgerald KA, Vogel SN. Phagosomal retention of Francisella tularensis results in TIRAP/Mal-independent TLR2 signaling. J Leukocyte Biology. 2009;87:275-81.
Gaston JS, Inman RD, Ryan ET, Venkatesan MM, Barry EM, Hale TL, Bourgeois AL, Walker RI. Vaccination of children in low-resource countries against Shigella is unlikely to present an undue risk of reactive arthritis. Vaccine. 2009;27:5432-5434.
Santiago AE, Cole LE, Franco A, Vogel SN, Levine MM, Barry EM. Characterization of rationally attenuated Francisella tularensis vaccine strains that harbor deletions in the guaA and guaB genes. Vaccine. 2009;27:2426-2436.
Baker K, Levine MM, Morison J, Phillips A, Barry EM. CfaE tip mutations in enterotoxigenic Escherichia coli CFA/I fimbriae fimbriae define critical human intestinal binding sites. Cellular Microbiology 2009;11:742-754.
Cole, LE, Santiago A, Barry E, Kang TJ, Shirey KA, Roberts ZJ, Elkins KL, Cross AS, Vogel SN. Macrophage proinflammatory response to Francisella tularensis live vaccine strain requires coordination of multiple signaling pathways. J Immunol. 2008;180:6885-6891.
Cole LE, Shirey KA, Barry EM, Santigao A, Rallabhandi P, Elkins KL, Puche A, Michalek, SM, Vogel SN. Toll-like receptor 2-mediated signaling requirements for Francisella tularensis live vaccine strain infection of murine macrophages. Infect Immun. 2007;75(8):4127-37.
Kotloff, KL, Simon JK, Pasetti MF, Sztein MB, Wooden S, Livio S, Blackwelder WC, Barry EM, Nataro JP, Picking WD, Levine MM. Safety and immunogenicity of CVD 1208S, a live, oral Shigella flexneri 2a vaccine grown on animal free media. Human Vaccines. 2007;3:268-275.
Levine MM, Kotloff KL, Barry EM, Pasetti MF, Sztein MB. Clinical trials of Shigella vaccines: two steps forward and one step back on a long, hard, road. Nat Rev Microbiol. 2007;5(7):540-553.
Barry E, Wang J, Wu T, Davis T, Levine MM. Immunogenicity of multivalent Shigella-ETEC candidate vaccine strains in a guinea pig mode. Vaccine. 2006;24:3727-34.
Kotloff, KL., Pasetti MF, Barry EM, Nataro JP, Wasserman SS, Sztein MB, Picking WD, Levine MM. Deletion of the Shigella enterotoxin genes further attenuates Shigella flexneri 2a bearing guanine auxotrophy in a Phase I trial of CVD 1204 and CVD 1208. J Infect Dis. 2004;190:1745-1754.
Pasetti MF, Barry EM, Losonsky G, Singh M, Medina-Moreno SM, Polo JM, Ulmer J, Robinson H, Sztein MB, Levine MM. Attenuated Salmonella enterica serovar Typhi and Shigella flexneri 2a strains mucosally deliver DNA vaccines encoding measles virus hemagglutinin, inducing specific immune responses and protection in cotton rats. J Virol. 2003;77:5209-5217.
Altboum Z, Levine MM, James E. Galen JE, Barry EM. Genetic characterization and immunogenicity of coli surface antigen 4 from enterotoxigenic Escherichia coli when it is expressed in a Shigella live-vector strain. Infect Immun. 2003;71:1352-1360.
Barry EM, Altboum Z, Losonsky G, Levine MM. Immune responses elicited against multiple enterotoxigenic Escherichia coli fimbriae and mutant LT expressed in attenuated Shigella vaccine strains. Vaccine. 2003;21:333-340.
Altboum Z, Barry EM, Losonsky G, Levine MM. Attenuated Shigella flexneri 2a ΔguaBA strain CVD 1204-expressing ETEC CS2 and CS3 fimbriae as a live mucosal vaccine against Shigella and enterotoxigenic Eschericia coli infection. Infect Immun. 2001;69:3150-3158.
Koprowski II H, Levine MM, Anderson R, Losonsky G, Pizza M, Barry EM. Attenuated Shigella flexneri 2a vaccine strain CVD 1204 expressing colonization factor antigen I (CFA/I) and mutant human heat labile toxin of enterotoxigenic Escherichia coli. Infect Immun. 2000;68:4884-4892.
Kotloff, KL, Noriega F, Samandari T, Sztein M, Losonsky G, Nataro J, Picking W, Barry EM, Levine MM. Shigella flexneri 2a strain CVD 1207 with specific deletions in virG, sen, set, and guaBA is highly attenuated in humans. Infect Immun. 2000;68:1034-1039.
Levine MM, Galen J, Barry E, Noriega F, Tacket C, Sztein M, Chatfield S, Dougan G, Losonsky G, Kotloff K. Attenuated Salmonella typhi and Shigella as live oral vaccines and as live vectors. Behring Inst Mitt. 1997 Feb;98:120-123.
Research Interests
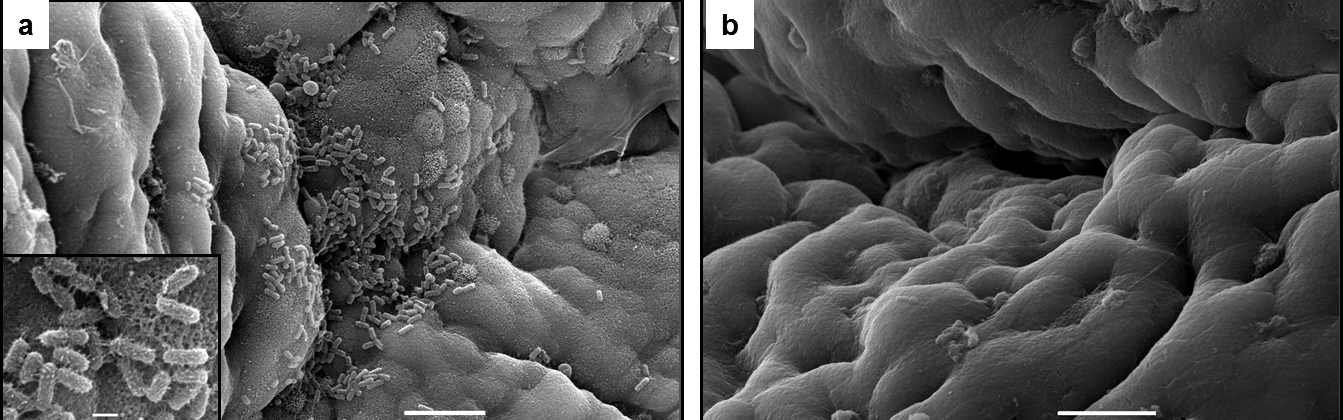
Laboratory projects include the study of basic mechanisms of pathogenesis of Shigella, ETEC and Francisella, antigen identification, host responses, and model development. Techniques include molecular engineering, electron microscopy, zero gravity organoid models, and in vitro organ culture (IVOC). As illustrated in the figure below, IVOC has been used to identify ETEC fimbrial epitopes critical for binding to pediatric intestinal tissue. Panel A shows wild type ETEC binding to pediatric intestinal biopsy tissue and panel B shows lack of binding of a fimbrial mutant. We have recently been funded to conduct studies on the pathogenesis of enteric bacteria using the human enteroid or “mini gut” model. This is a recent breakthrough technology that utilizes normal human biopsy samples that are differentiated in multicellular enteroids that represent the most human-relevant system for studying human-specific pathogens.
Awards and Affiliations
2015: University System of Maryland PROMISE Alliance for Graduate Education and the Professoriate Outstanding Faculty Mentor (facilitating development of underrepresented minority graduate students in STEM fields)
2011: Faculty Teacher of the Year Award, Department of Medicine, Division of Geographic Medicine, UMSOM
2009/2012: Teaching Commendation in Host Defenses and Infectious Diseases Bacteriology/Mycology Section
Grants and Contracts
James Kaper, Ph.D. (PI)
Eileen Barry, Ph.D. (PI Project 2)
P01AI125181
7/1/16-6/30/2021
National Institutes of Health (NIH)/National Institute of Allergy and Infectious Diseases (NIAID)
Pathogenesis of E. Coli and Shigella Infections in Human Enteroid Models
Marcela Pasetti, Ph.D. (PI)
Eileen Barry, Ph.D. (Co-I)
R01AI125841
NIH/NIAID
4/1/16-3/31/2021
Serological Assays to Predict Shigella Vaccine Efficacy
Eileen Barry, Ph.D. (PI; MPI K. Hazlett, D. Reed)
R01AI123129
2/15/2016-1/31/2021
NIH NIAID
Correlates of Vaccine Induced, Tunable Protection in an Outbred Tularemia Model
Eileen Barry, Ph.D. (PI)
9/1/2015-8/31/2017
PATH-EVI
Shigella Micro-Array Development
Marcela Pasetti, Ph.D. (PI)
Eileen Barry, Ph.D. (Co-I)
1R01AI117734-01
4/1/2015-3/31/2020
NIH/NIAID
Vaccines and Maternally Acquired Immunity to Prevent Shigellosis in Children
Myron Levine, M.D., D.T.P.H. (PI)
Eileen Barry, Ph.D. (PI Project 3)
U19AI109776
3/01/2014-2/28/2019
NIH
Immunoprophylactic Strategies to Control Emerging Enteric Infections
Project 3: Shigella Live Vector-Based Multivalent Vaccine
Eileen Barry, Ph.D. (PI)
1R01AI102966
3/15/2013-3/14/2017
NIH/NIAID
Advancement of a Defined, Protective, Live Attenuated Tularemia Vaccine
Lab Techniques and Equipment
BSL2 and BSL3 laboratories, ABSL2 and ABSL3 laboratories, genetic engineering of pathogenic bacteria, organoid and enteroid models, various cell culture assays, genomics and transcriptomics.
Administrative and Institutional Services
2007-present: Member, Graduate Student Admissions Committee, GPILS Molecular Microbiology and Immunology Program
2013-present: Member, Department of Medicine Appointment, Promotion and Tenure Committee
International and National Service
1999-present: Member, Military Infectious Diseases Study Section
2000: Member, NIH Special Emphasis Panel: Innovative Research in Human Mucosal Immunity Study Section
2003: Temporary Advisor, World Health Organization: Future Directions of Research on ETEC Vaccines for Children in Developing Counties
2003: Panel Member, Joint Medical Technology Workshop, Military Infectious Disease: Diarrhea and other Bacterial Diseases
2004: Member, NIH Special Emphasis Panel: Biodefense and Emerging Infectious Disease Research Opportunities Study Section (1/2004 and 12/2004)
2004: Temporary Advisor, World Health Organization, Future Needs and Directions for Shigella Vaccines
2004: Member, NIH Special Emphasis Panel: In Vitro and Animal Models for Emerging Infectious Diseases and Biodefense Study Section
2005: Member, NIH Special Emphasis Panel: Disabling Innate Immune Evasion: New Attenuated Vaccines Study Section
2005-2010: Member, NIH Special Emphasis Panel: Small Business Grant Applications: Microbial Vaccine Development Study Section (7/2005, 11/2005, 7/2006, 10/2006, 7/2007, 2/2008, 6/2008, 10/2008, 2/2009, 7/2009, 2/2010)
2005: Member, Cooperative Grants Program of the US Civil Research and Development Foundation Study Section
2005-2010: Advisor, Scientific Working Group of the PathoSystems Resource Integration Center of the Virginia Bioinformatics Institute at Virginia Polytechnic Institute
2005-present: Ad Hoc Reviewer, Microbial Pathogenesis, Journal of Immunology, Journal of Bacteriology, Clinical and Experimental Immunology, FEMS Immunology and Medical Microbiology, Vaccinology, Vaccine, Microbes and Infection, FEMS Microbiology Letters, Infection and Immunity, Journal of Medical Microbiology, J. Biol. Chem., PNAS, Pathogens and Disease
2006: Member, NIH Special Emphasis Panel: Cooperative Research Partnerships for Biodefense Study Section (1/2006, 2/2006)
2008: Panel Member, Program for Appropriate Technology in Health, Enterics Vaccine Initiative Reactive Arthritis Workshop
2010: Member, NIH Partnerships for Biodefense (Vaccines/Immunotherapeutics) Study Section
2012-2016: Permanent member, NIH NIAID Host Interactions with Bacterial Pathogens (HIBP) Study Section
2014 – 2016: Member, editorial board, Clinical and Vaccine Immunology
