May 10, 2023 | Vanessa McMains
In an effort to determine the cause behind a rare condition that causes heart failure in children, University of Maryland School of Medicine researchers have identified new gene mutations responsible for the disorder in an infant patient. They were then able to learn how the mutation works and used a drug to reverse its effects in heart muscle cells derived from stem cells from the patient.
 The findings, published in late April in Circulation, suggest that treatments could be developed to manage the condition rather than requiring a heart transplant, which is the standard treatment for this condition in children.
The findings, published in late April in Circulation, suggest that treatments could be developed to manage the condition rather than requiring a heart transplant, which is the standard treatment for this condition in children.
“Although much has been studied about heart failure in adults, there is still much to learn about the genetic causes of heart failure in infants,” said Charles “Chaz” Hong, MD, PhD, Melvin Sharoky, MD Professor of Medicine and Physiology, Director of Cardiology Research, and Co-Chief of Cardiovascular Medicine at UMSOM. “Mutations in the gene we identified had been implicated in microcephaly in babies but not yet in human heart disease.”
Infantile dilated cardiomyopathy is a common cause of heart failure — responsible for about half of pediatric heart failure cases — whose cause is most often unknown. Although relatively rare, occurring in about one in 200,000 births, infants with the condition have hearts that fail to contract as effectively, so they are not able to pump as much blood as they should.
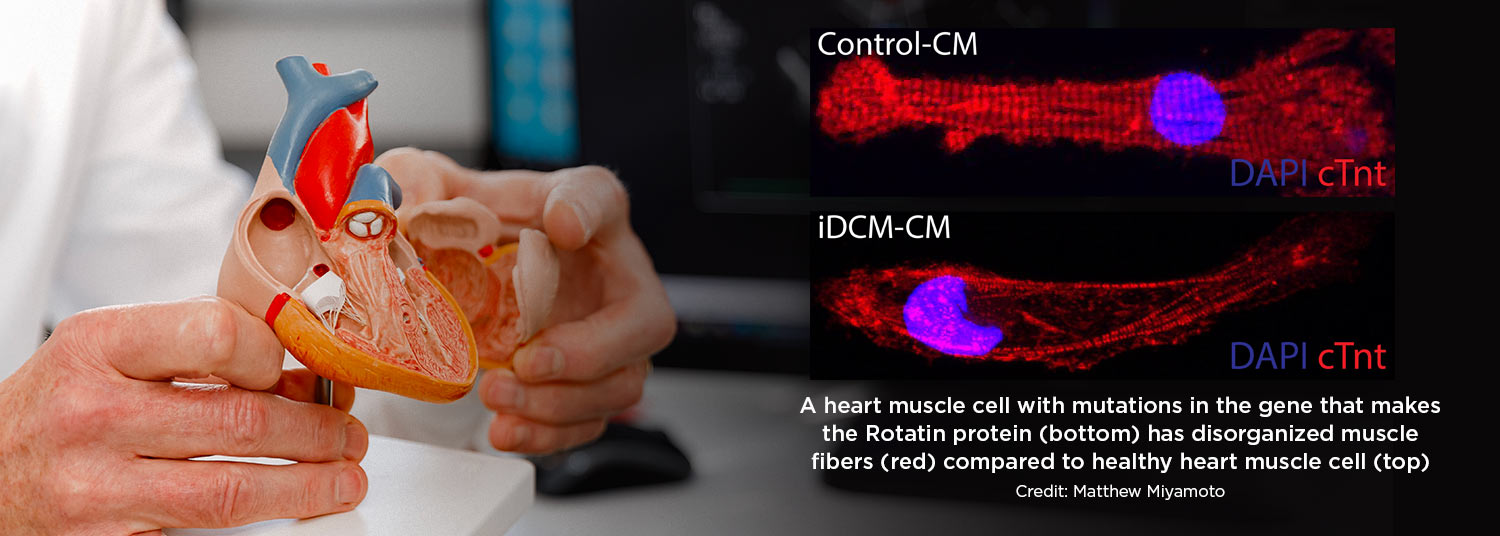
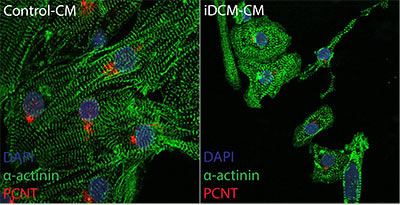 This genetic mutation discovered by Dr. Hong and his colleagues was found to normally make a protein found in a cell structure, the centrosome, that functions as a tether for the cell’s skeleton and is best known for its role during cell division.
This genetic mutation discovered by Dr. Hong and his colleagues was found to normally make a protein found in a cell structure, the centrosome, that functions as a tether for the cell’s skeleton and is best known for its role during cell division.
Without this protein, muscle cells in the heart were unable to organize themselves neatly and did not contract as well, which in turn affected the heart’s pumping, the researchers theorized.
“We originally dismissed our findings as artifacts that the cell division machinery would be involved in this kind of heart muscle dysfunction,” said Dr. Hong. “We thought that once the heart cells matured, this cell division machinery completely disappeared, but it turned out, it moves to a new location in the cell and takes on a new role in heart muscle function.”
 To identify this gene mutation responsible for infant heart failure, the researchers removed a sample of heart cells from the patient’s diseased heart after it was removed during a transplant. They then converted this heart tissue to stem cells, so they could grow more cells and study them in the lab. They determined that the patient had two different mutations of a gene, one from each parent, that normally encodes for the Rotatin protein.
To identify this gene mutation responsible for infant heart failure, the researchers removed a sample of heart cells from the patient’s diseased heart after it was removed during a transplant. They then converted this heart tissue to stem cells, so they could grow more cells and study them in the lab. They determined that the patient had two different mutations of a gene, one from each parent, that normally encodes for the Rotatin protein.
When the researchers then conducted an experiment to remove this same protein from zebrafish hearts, these hearts developed with signs of heart failure. The researchers also looked at fruit fly hearts missing Rotatin and saw that the muscle cells in these hearts were disorganized and did not contract as well as they should, similar to what happens in infant hearts with the disorder.
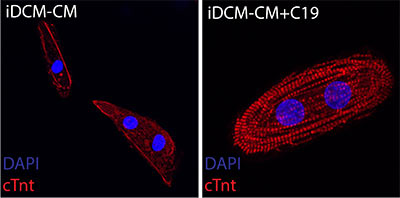
“This is the first human disease known to be caused by disrupting the transition in centrosome structure which normally occurs shortly after birth,” said Matthew Miyamoto, the first co-author who worked on this project as a rising second-year medical student in Dr. Hong’s laboratory. The researchers then used the drug C19 that was known to organize centrosomes in developing heart muscle cells derived from the patient with infantile dilated cardiomyopathy. The drug restored organization of the developing heart muscle cells grown in a dish from the patient’s stem cells and their ability to contract.
 “Because centrosomes play such a fundamental role in heart muscle development, specifically cell replication, structure, and function, a better understanding of this tissue-specific programmed process will be highly relevant to future cardiac regenerative therapy efforts,” said UMSOM Dean, Mark T. Gladwin, MD, who is also Vice President for Medical Affairs, University of Maryland, Baltimore (UMB), and the John Z. and Akiko K. Bowers Distinguished Professor.
“Because centrosomes play such a fundamental role in heart muscle development, specifically cell replication, structure, and function, a better understanding of this tissue-specific programmed process will be highly relevant to future cardiac regenerative therapy efforts,” said UMSOM Dean, Mark T. Gladwin, MD, who is also Vice President for Medical Affairs, University of Maryland, Baltimore (UMB), and the John Z. and Akiko K. Bowers Distinguished Professor.
Dr. Hong added, “It is only through collaborations between cardiologists, medical student trainees, and laboratory researchers that allowed this biomedical discovery which we hope will one day translate to medical treatments for children with this condition.”
Patrice Desvigne-Nickens, MD, a medical officer in the Heart Failure and Arrhythmias Branch in the Division of Cardiovascular Sciences at the National Heart, Lung, and Blood Institute (NHLBI), part of the National Institutes of Health, agreed. “This study makes an important contribution toward understanding the biological underpinnings of infantile dilated cardiomyopathy and its relationship to heart failure,” she said. “We look forward to future studies to clarify and confirm these findings in an effort to improve heart failure outcomes.”
This study was funded by grants from the NHLBI (R01HL135129), the Maryland Stem Cell Research Fund (HP-00089001), and an AOA Carolyn L. Kuckein Student Research Fellowship.
The authors have filed a pending patent on using C19 to treat infantile dilated cardiomyopathy. In accordance with UMB policy, the authors have disclosed their interest in the patent, and the university is managing this relationship to ensure objectivity in the research.
DISCLAIMER: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
About the University of Maryland School of Medicine
Now in its third century, the University of Maryland School of Medicine was chartered in 1807 as the first public medical school in the United States. It continues today as one of the fastest growing, top-tier biomedical research enterprises in the world — with 46 academic departments, centers, institutes, and programs, and a faculty of more than 3,000 physicians, scientists, and allied health professionals, including members of the National Academy of Medicine and the National Academy of Sciences, and a distinguished two-time winner of the Albert E. Lasker Award in Medical Research. With an operating budget of more than $1.3 billion, the School of Medicine works closely in partnership with the University of Maryland Medical Center and Medical System to provide research-intensive, academic, and clinically based care for nearly 2 million patients each year. The School of Medicine has nearly $600 million in extramural funding, with most of its academic departments highly ranked among all medical schools in the nation in research funding. As one of the seven professional schools that make up the University of Maryland, Baltimore campus, the School of Medicine has a total population of nearly 9,000 faculty and staff, including 2,500 students, trainees, residents, and fellows. The combined School of Medicine and Medical System (“University of Maryland Medicine”) has an annual budget of over $6 billion and an economic impact of nearly $20 billion on the state and local community. The School of Medicine, which ranks as the 8th highest among public medical schools in research productivity (according to the Association of American Medical Colleges profile) is an innovator in translational medicine, with 606 active patents and 52 start-up companies. In the latest U.S. News & World Report ranking of the Best Medical Schools, published in 2021, the UM School of Medicine is ranked #9 among the 92 public medical schools in the U.S., and in the top 15 percent (#27) of all 192 public and private U.S. medical schools. The School of Medicine works locally, nationally, and globally, with research and treatment facilities in 36 countries around the world. Visit medschool.umaryland.edu
Contact
Vanessa McMains
Director, Media & Public Affairs
University of Maryland School of Medicine
Institute of Human Virology
vmcmains@ihv.umaryland.edu
Cell: 443-875-6099
Related stories

Tuesday, January 24, 2023
Special Vascular Cells Adjust Blood Flow in Brain Capillaries Based on Local Energy Needs
When we smell hot dogs, it may trigger memories of backyard barbeques or attending baseball games during childhood. During this process, the areas of the brain that control smell and long-term memory are rapidly firing off impulses. To fuel these signals from neurons, the active brain regions need oxygen and energy in the form of blood sugar glucose, which is quickly delivered through blood vessels.

Wednesday, February 02, 2022
ADHD Medicine May Treat Symptoms of Genetic Movement Disorder in Children, University of Maryland School of Medicine Study Finds
Using a common attention deficit hyperactivity disorder (ADHD) medication appears to help manage the symptoms of a rare and currently difficult to treat genetic movement disorder primarily found in children, according to a new study from a University of Maryland School of Medicine (UMSOM) researcher Andrea Meredith, PhD, and her collaborators.

Thursday, October 21, 2021
New Research Finds Air Pollution Reduces Sperm Counts through Brain Inflammation
Researchers have long known that air pollution can increase the risk of disorders such as obesity, diabetes, and fertility, but they did not know the exact mechanism for how it can lead to these health conditions.
Friday, April 09, 2021
Researchers Link Several Heart Disease Risk Factors to Increased Risk of COVID-19 Infections
As the COVID-19 pandemic lingers, researchers have found associations between certain lifestyle factors and a person’s risk of getting infected. While it has already been established that those with Type II diabetes and a high body mass index (BMI) are at greater risk of experiencing hospitalizations and other severe complications related to COVID-19, they are also at greater risk of getting symptomatic infection in the first place. That is the finding of a recent study conducted by researchers at the University of Maryland School of Medicine (UMSOM)that was published in the journal, PLoS ONE.

Tuesday, October 15, 2019
Theme of 'Courage, Hope and Faith' Highlights Investiture of Dr. Charles Hong as the Melvin Sharoky, MD, Professor of Medicine
In a moving address to an audience of family members, UMSOM faculty and staff, and distinguished invited guests, Charles Hong, MD, PhD, echoed a theme that was heard throughout the ceremony of his investiture as the Melvin Sharoky, MD, Professor of Medicine: “Courage, Hope and Faith.”
