February 22, 2023 | Deborah Kotz
Study Led to FDA Approval of Focused Ultrasound Device that Provides Novel Treatment Option Without Requiring Incision
Patients with Parkinson’s disease achieved a significant improvement in their tremors, mobility, and other physical symptoms after having a minimally-invasive procedure involving focused ultrasound, according to a new study today published in the New England Journal of Medicine.
 The clinical trial was led by researchers at the University of Maryland School of Medicine (UMSOM) and involved 94 Parkinson’s disease patients who were randomly assigned to undergo focused ultrasound to ablate a targeted region on one side of the brain or to have a sham procedure. Nearly 70 percent of patients in the treatment group were considered successful responders to treatment after three months of follow-up, compared to 32 percent in the control group who had an inactive procedure without focused ultrasound.
The clinical trial was led by researchers at the University of Maryland School of Medicine (UMSOM) and involved 94 Parkinson’s disease patients who were randomly assigned to undergo focused ultrasound to ablate a targeted region on one side of the brain or to have a sham procedure. Nearly 70 percent of patients in the treatment group were considered successful responders to treatment after three months of follow-up, compared to 32 percent in the control group who had an inactive procedure without focused ultrasound.
Two-thirds of those who responded initially to the focused ultrasound treatment continued to have a successful response from the treatment a year later.
Patients were treated at the University of Maryland Medical Center (UMMC), the academic flagship hospital for the University of Maryland Medical System, and 15 other sites in North America, Asia, and Europe.
“These results are very promising and offer Parkinson’s disease patients a new form of therapy to manage their symptoms. There is no incision involved, which means no risk of a serious infection or brain bleeding,” said study corresponding author Howard Eisenberg, MD, the Raymond K. Thompson Professor of Neurosurgery at UMSOM and a neurosurgeon at UMMC.
About one million Americans have Parkinson’s disease, a neurodegenerative disorder that affects brain cells or neurons in a specific area of the brain that produce the brain chemical dopamine. Symptoms include shaking, stiffness, and difficulty with balance and coordination. Other treatments for Parkinson’s include medications and deep brain stimulation (DBS) from surgically implanted electrodes. The medications can cause involuntary, erratic movements called dyskinesia as doses are increased to control symptoms. Usually offered when medications fail, DBS involves brain surgery to insert the electrodes through two small openings in the skull. The procedure carries a small risk of serious side effects including brain hemorrhage and infection.
 “Our study will help doctors and patients make an informed decision when considering this new treatment modality to help better manage symptoms,” said study co-author Paul Fishman, MD, PhD, Professor of Neurology at UMSOM and a neurologist at UMMC. “But it’s important for patients to realize that none of the treatments currently available will cure Parkinson’s disease.”
“Our study will help doctors and patients make an informed decision when considering this new treatment modality to help better manage symptoms,” said study co-author Paul Fishman, MD, PhD, Professor of Neurology at UMSOM and a neurologist at UMMC. “But it’s important for patients to realize that none of the treatments currently available will cure Parkinson’s disease.”
Focused ultrasound is an incisionless procedure, performed without the need for anesthesia or an in-patient stay in the hospital. Patients, who remain fully alert, lie in a magnetic resonance imaging (MRI) scanner, wearing a transducer helmet. Ultrasonic energy is targeted through the skull to the globus pallidus, a structure deep in the brain that helps control regular voluntary movement. MRI images provide doctors with a real-time temperature map of the area being treated, to precisely pinpoint the target and to apply a high enough temperature to ablate it. During the procedure, the patient is awake and providing feedback, which allows doctors to monitor the immediate effects of the tissue ablation and make adjustments as needed.
The device, called Exablate Neuro, was approved over a year ago by the US Food and Drug Administration (FDA) to treat advanced Parkinson’s disease on one side of the brain. The FDA approval was based on findings from the UMSOM clinical trial published today. It is now widely available at UMMC. However, it is not yet covered by insurance, including Medicare, so patients currently need to pay out of pocket for the procedure.
“Focused ultrasound is only approved by the FDA to treat one side of the brain in Parkinson’s disease patients, so it may be more appropriate at this time for patients with symptoms predominantly on one side,” said study co-author Vibhor Krishna, MD, a professor of neurosurgery at the University of North Carolina, Chapel Hill.
Diagnosed with Parkinson’s disease in 2020, Melanie Carlson, a 41-year-old mother of a toddler, found that the medications she was taking to manage the condition caused her to have uncontrollable shaking. Her symptoms were so severe, she was dependent on a walker and unable to take her daughter to the playground. Last June, she opted to have focused ultrasound at UMMC after learning about the FDA approval.
Resources for the Media:
“Focused ultrasound was really transformative. So many of my fine motor skills have returned. I’m putting on eyeliner again and taking showers again without falling,” Carlson said. “This honestly feels like one of the best years of my life. I just feel so fortunate. I hope more people can benefit from this procedure.”
Patients in the trial -- with moderate Parkinson’s who were not responding well to medications -- were treated with one session of focused ultrasound on the side of their brain that controlled the side of their body where symptoms were more severe. The study was designed as a crossover trial, where 25 patients in the control group were offered the active treatment three months after their sham procedure; 20 out of 25 opted to have the focused-ultrasound treatment and experienced similar benefits as the initial treatment group.
 Those in the treatment group had an immediate improvement of at least three points on a standard assessment -- measuring tremors, walking abilities, and rigidity in the legs and arms -- compared to an 0.3 point improvement in the control group. They also experienced relief from side effects from Parkinson’s medications. They were assessed again at three months and at 12 months. Patients will continue to be followed for five years to evaluate how long the treatment lasts and progression of the disease.
Those in the treatment group had an immediate improvement of at least three points on a standard assessment -- measuring tremors, walking abilities, and rigidity in the legs and arms -- compared to an 0.3 point improvement in the control group. They also experienced relief from side effects from Parkinson’s medications. They were assessed again at three months and at 12 months. Patients will continue to be followed for five years to evaluate how long the treatment lasts and progression of the disease.
Adverse events from the procedure included headache, dizziness, and nausea that resolved within a day or two of treatment. Some patients experienced mild side effects from the focused ultrasound treatment, including slurred speech, walking issues, and loss of taste. These usually resolved within the first few weeks.
Dr. Eisenberg and his colleagues are currently conducting a clinical trial to test the Exablate Neuro device on both sides of the brain, delivering focused ultrasound treatments in two sessions, six months apart. “So far, we’ve had promising results,” Dr. Eisenberg said.
The study was funded by Insightec, manufacturer of Exablate Neuro.
 “We are on the edge of the frontier with focused ultrasound, as ongoing research evaluates the procedure in different brain areas affected by Parkinson’s, such as the subthalamic nucleus, which controls movement regulation,” said UMSOM Dean, Mark T. Gladwin, MD, who is also Vice President for Medical Affairs, University of Maryland, Baltimore, and the John Z. and Akiko K. Bowers Distinguished Professor. “Researchers also are studying how focused ultrasound could be used to temporarily open the blood-brain barrier to help experimental Parkinson’s treatments, like immunotherapy, get into the brain more easily.”
“We are on the edge of the frontier with focused ultrasound, as ongoing research evaluates the procedure in different brain areas affected by Parkinson’s, such as the subthalamic nucleus, which controls movement regulation,” said UMSOM Dean, Mark T. Gladwin, MD, who is also Vice President for Medical Affairs, University of Maryland, Baltimore, and the John Z. and Akiko K. Bowers Distinguished Professor. “Researchers also are studying how focused ultrasound could be used to temporarily open the blood-brain barrier to help experimental Parkinson’s treatments, like immunotherapy, get into the brain more easily.”
Added Bert W. O'Malley, MD, President and CEO of UMMC, “As home to one of the top movement disorder centers in the country, and one of only a few medical centers that offer focused ultrasound for Parkinson’s, we see firsthand the technology’s impact on people’s lives. Congratulations to the researchers—and the trial participants—for exemplifying the innovation and discovery that will make focused ultrasound available to more people in the years to come.”
About the University of Maryland School of Medicine
Now in its third century, the University of Maryland School of Medicine was chartered in 1807 as the first public medical school in the United States. It continues today as one of the fastest growing, top-tier biomedical research enterprises in the world -- with 46 academic departments, centers, institutes, and programs, and a faculty of more than 3,000 physicians, scientists, and allied health professionals, including members of the National Academy of Medicine and the National Academy of Sciences, and a distinguished two-time winner of the Albert E. Lasker Award in Medical Research. With an operating budget of more than $1.3 billion, the School of Medicine works closely in partnership with the University of Maryland Medical Center and Medical System to provide research-intensive, academic, and clinically based care for nearly 2 million patients each year. The School of Medicine has nearly $600 million in extramural funding, with most of its academic departments highly ranked among all medical schools in the nation in research funding. As one of the seven professional schools that make up the University of Maryland, Baltimore campus, the School of Medicine has a total population of nearly 9,000 faculty and staff, including 2,500 students, trainees, residents, and fellows. The combined School of Medicine and Medical System (“University of Maryland Medicine”) has an annual budget of over $6 billion and an economic impact of nearly $20 billion on the state and local community. The School of Medicine, which ranks as the 8th highest among public medical schools in research productivity (according to the Association of American Medical Colleges profile) is an innovator in translational medicine, with 606 active patents and 52 start-up companies. In the latest U.S. News & World Report ranking of the Best Medical Schools, published in 2021, the UM School of Medicine is ranked #9 among the 92 public medical schools in the U.S., and in the top 15 percent (#27) of all 192 public and private U.S. medical schools. The School of Medicine works locally, nationally, and globally, with research and treatment facilities in 36 countries around the world. Visit medschool.umaryland.edu
Contact
Deborah Kotz
Senior Director of Media Relations
Office of Public Affairs & Communications
University of Maryland School of Medicine
Email: DKotz@som.umaryland.edu
o: 410-706-4255
c: 410-804-0054
t: @debkotz2
Related stories

Monday, February 28, 2022
UM School of Medicine Research Leads to Innovative Non-Invasive Treatment for Parkinson’s Disease at UM Medical Center
– A non-invasive ultrasound treatment for Parkinson’s disease that was tested in a pivotal trial led by University of Maryland School of Medicine (UMSOM) researchers is now broadly available at the University of Maryland Medical Center (UMMC). The device, called Exablate Neuro, was approved in November by the US Food and Drug Administration to treat advanced Parkinson’s disease on one side of the brain.
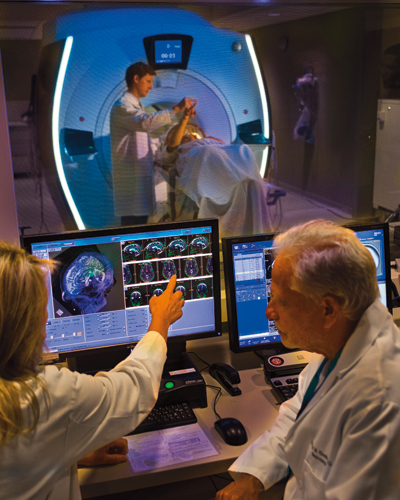
Tuesday, August 30, 2016
New Study Shows Breakthrough Treatment for Essential Tremor Using Focused Ultrasound
University of Maryland School of Medicine (UM SOM) researchers, along with an international group of investigators, have discovered for the first time that treatment with MRI-guided focused ultrasound can effectively treat patients with essential tremor (ET), a neurological movement disorder that affects an estimated 10 million people in the U.S. The results, which are published in the forthcoming issue of the New England Journal of Medicine, led to recent approval of the treatment by the Food & Drug Administration (FDA),

