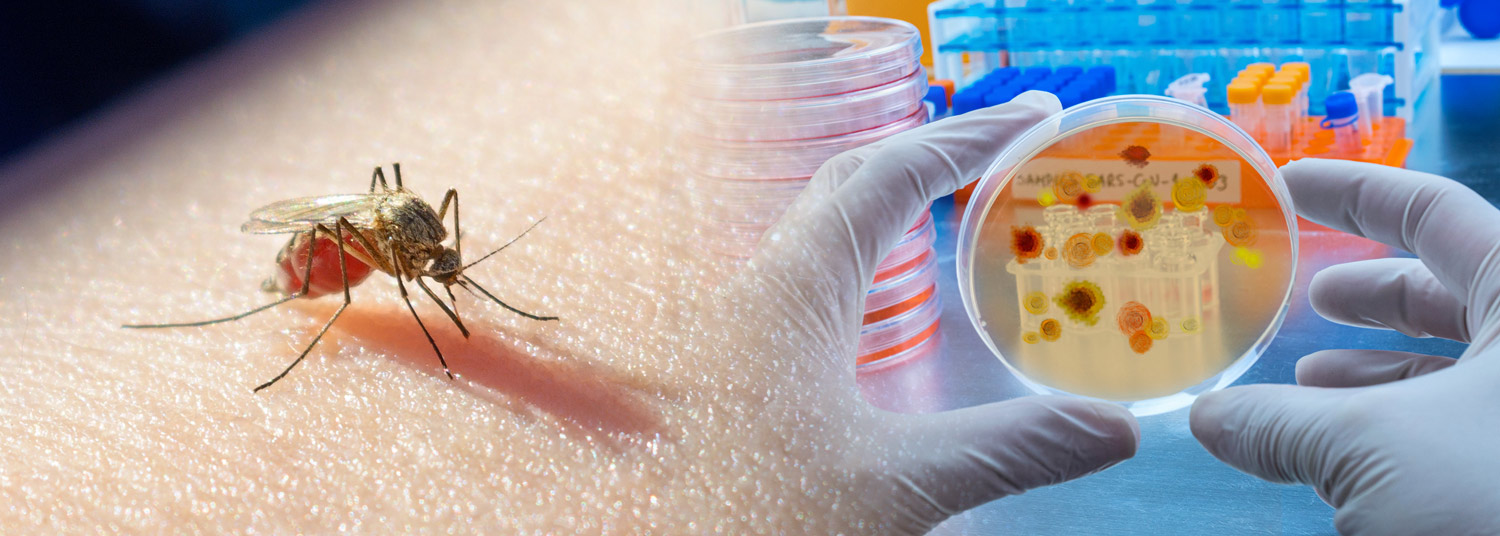
July 18, 2022 | Heide Aungst
UMSOM Research Could Guide Future Vaccine Development
Malaria is the deadliest mosquito-borne parasitic infection of humans. In 2021, after a century of research, the World Health Organization (WHO) approved the world’s first malaria vaccine. That vaccine reduces the incidence of malaria infections in young children aged 5-17 months by only 30 percent, meaning that it remains critical to continue developing and testing more effective vaccines.
WHO’s goal is to find a vaccine that prevents infection as well as cases of severe malaria. However, testing vaccines in the field is challenging and requires large number of volunteers and long periods of follow-up. This process increases the expense and reduces the number of trials that researchers can perform.
 Now, scientists at the University of Maryland School of Medicine’s (UMSOM) Institute for Genome Sciences (IGS) and the UMSOM Center for Vaccine Development and Global Health (CVD), and their collaborators report a new way to test vaccines that may be as rigorous and stringent as exposure to field strains of malaria. Their study was published in the June issue of Nature Communications.
Now, scientists at the University of Maryland School of Medicine’s (UMSOM) Institute for Genome Sciences (IGS) and the UMSOM Center for Vaccine Development and Global Health (CVD), and their collaborators report a new way to test vaccines that may be as rigorous and stringent as exposure to field strains of malaria. Their study was published in the June issue of Nature Communications.
Their method has two key aspects. First, they expose vaccinated volunteers to malaria in a controlled clinical environment. Secondly, for this testing, they use a strain of malaria that is genetically very different from the one used in the vaccine, as well as from strains in the geographic region to which the vaccine is intended.
This technique allows scientists to test how well the vaccine works in small numbers of volunteers under controlled settings and in a rapid fashion and predicts how well the vaccine may perform in the field. This lets researchers select the best vaccines for larger studies in the field. This method will increase the efficiency of vaccine testing and should accelerate malaria vaccine development.
“The standard for many investigators has been to test vaccines with a strain similar to the one used in the vaccine’s development,” explained the study’s lead author, Joana Carneiro da Silva, PhD, Professor of Microbiology and Immunology at UMSOM and IGS. “Using a strain that is both genetically distant from the one in the vaccine -- as well as from the strains circulating in the area where malaria is rampant and the vaccine will be used– is a more stringent way to test vaccine effectiveness.”
Researchers are studying the effectiveness of a vaccine (PfSPZ Vaccine) made by the company Sanaria, Inc, based in Rockville, Maryland. This vaccine uses the West African parasite strain known as PfNF54. One objective is to use this vaccine to protect individuals with little or no previous exposure to malaria, including those living or traveling in Africa. The long-term goal is to use the vaccine in mass vaccination programs to eliminate malaria from specific regions in Africa.
For the study, researchers infected mosquitoes with a Brazilian malarial strain and then exposed U.S. volunteers who had been vaccinated with the Sanaria vaccine (as well as those who received a placebo) to the bites of infected mosquitos in a controlled clinical setting. They also vaccinated research participants in Mali with the same dose of the vaccine to compare observed vaccine efficacy in the field with that in the clinic. Through genomic sequencing, researchers had shown that the Brazilian strain differed greatly from 700 strains previously collected from across Africa, including the one used to make the vaccine.
 The researchers looked at about 200 volunteers in four trials – two in the United States and two in Mali. In all four, they observed how many people became infected with malaria, as well as how long it took for them to become infected, comparing clinic to field. At the end of six months, they found the vaccine was just as effective in both populations.
The researchers looked at about 200 volunteers in four trials – two in the United States and two in Mali. In all four, they observed how many people became infected with malaria, as well as how long it took for them to become infected, comparing clinic to field. At the end of six months, they found the vaccine was just as effective in both populations.
In addition, previous comparisons had shown that those volunteers who had never been exposed to malaria developed more antibodies than those in the field, proving that it would work well in first-time travelers to the area.
The research team included scientists from Sanaria Inc.; the Naval Medical Research Center; University of Tübingen in Germany; the Malaria Research and Training Center in Bamako, Mali; and the Laboratory of Malaria Immunology and Vaccinology at NIAID, NIH.
The World Health Organization estimates that in 2020, 241 million cases and 627,000 deaths worldwide were due to malaria, including 2,000 cases diagnosed in the United States from travelers and immigrants who were exposed elsewhere. The variety of malarial strains globally makes vaccine development particularly difficult.
“Given the diversity of malaria strains worldwide, this research demonstrates that the Brazilian strain is as diverse as any strain detected in Africa,” said Kirsten E. Lyke, MD, Professor of Medicine and Director of the Malaria Vaccine and Challenge Unit at the CVD and an author of the paper.
 Dr. da Silva noted this new model can be used in the future in selecting challenge strains to test the efficacy of vaccines against other parasitic diseases, using carefully controlled clinical settings to compare against field studies.
Dr. da Silva noted this new model can be used in the future in selecting challenge strains to test the efficacy of vaccines against other parasitic diseases, using carefully controlled clinical settings to compare against field studies.
Albert Reece, MD, PhD, MBA, Executive Vice President for Medical Affairs, UM, Baltimore, the John Z. and Akiko K. Bowers Distinguished Professor, and Dean at the University of Maryland School of Medicine, said, “The world has waited decades for effective malaria vaccines. Dr. Silva and her colleagues have found a way to greatly expedite our ability to examine potential vaccine formulations in clinic to identify the most promising candidates. This study also shows how important genomics are to help establish the efficacy of new vaccine formulations and to guide vaccine development.”
Funding
This study was funded the National Institutes of Health’s National Institute of Allergy and Infectious Diseases intramural program and grants (U19AI110820, R01AI141900, 5R44AI058375-10, and 5R44AI055229-11) , and the Department of Defense (W81XWH1420011).
Authors from the University of Maryland School of Medicine
Joana C. da Silva, PhD, Professor, Microbiology and Immunology; Associate Professor, Institute for Genome Sciences
Ankit Dwivedi, PhD, Bioinformatics Analyst, Institute for Genome Sciences
Kara A. Moser, PhD, previous doctoral student, Institute for Genome Sciences
Kirsten E. Lyke, MD, Professor of Medicine and Director, Malaria Vaccine and Challenge Unit Center for Vaccine Development and Global Health
Author Conflicts of Interest
Competing interests Thomas L. Richie, Tooba Murshedkar B. Kim Lee Sim, and Stephen L. Hoffman are salaried employees of Sanaria Inc., the developer, and owner of PfSPZ Vaccine and PfSPZ Challenge (7G8). In addition, Hoffman and Sim. have a financial interest in Sanaria Inc. No other authors declare competing interests.
About the University of Maryland School of Medicine
Now in its third century, the University of Maryland School of Medicine was chartered in 1807 as the first public medical school in the United States. It continues today as one of the fastest growing, top-tier biomedical research enterprises in the world -- with 46 academic departments, centers, institutes, and programs, and a faculty of more than 3,000 physicians, scientists, and allied health professionals, including members of the National Academy of Medicine and the National Academy of Sciences, and a distinguished two-time winner of the Albert E. Lasker Award in Medical Research. With an operating budget of more than $1.3 billion, the School of Medicine works closely in partnership with the University of Maryland Medical Center and Medical System to provide research-intensive, academic and clinically based care for nearly 2 million patients each year. The School of Medicine has nearly $600 million in extramural funding, with most of its academic departments highly ranked among all medical schools in the nation in research funding. As one of the seven professional schools that make up the University of Maryland, Baltimore campus, the School of Medicine has a total population of nearly 9,000 faculty and staff, including 2,500 students, trainees, residents, and fellows. The combined School of Medicine and Medical System (“University of Maryland Medicine”) has an annual budget of over $6 billion and an economic impact of nearly $20 billion on the state and local community. The School of Medicine, which ranks as the 8th highest among public medical schools in research productivity (according to the Association of American Medical Colleges profile) is an innovator in translational medicine, with 606 active patents and 52 start-up companies. In the latest U.S. News & World Report ranking of the Best Medical Schools, published in 2021, the UM School of Medicine is ranked #9 among the 92 public medical schools in the U.S., and in the top 15 percent (#27) of all 192 public and private U.S. medical schools. The School of Medicine works locally, nationally, and globally, with research and treatment facilities in 36 countries around the world. Visit medschool.umaryland.edu
About the Institute for Genome Sciences
The Institute for Genome Sciences (IGS) at the University of Maryland School of Medicine has revolutionized genomic discoveries in medicine, agriculture, environmental science, and biodefense since its founding in 2007. IGS investigators research areas of genomics and the microbiome to better understand health and disease, including treatments, cures, and prevention. IGS investigators also lead the development of the new field of microbial forensics. IGS is a leading center for major biological initiatives currently underway including the NIH-funded Human Microbiome Project (HMP) and the NIAID-sponsored Genomic Sequencing Center for Infectious Diseases (GSCID). Follow us on Twitter @GenomeScience.
Contact
Heide Aungst
HAungst@som.umaryland.edu
216-970-5773 (cell)
Related stories

Friday, October 17, 2025
New Monoclonal Antibody Shows Promise for Preventing Malaria Infections
Malaria remains one of the leading causes of death among children in sub-Saharan Africa, claiming more than 600,000 lives each year worldwide with limited efficacy in currently available treatments and vaccines. Now a new early-stage clinical trial found that a novel monoclonal antibody provided dose-dependent full protection against the malaria parasite with minimal side effects.

Friday, July 25, 2025
New Research Simulates Cancer Cell Behavior
In the same vein as weather forecast models that predict developing storms, researchers now have developed a method to predict the cell activity in tissues over time. The new software combines genomics technologies with computational modeling to predict cell changes in behavior, such as communication between cells that could cause cancer cells to flourish.

Wednesday, November 06, 2024
Genomic Databases Need More Diversity
It is commonly known that most genomic databases are biased toward people with European ancestry. Scientists have warned that leaving out other populations could skew results in areas such as drug development, diagnostic testing, and polygenic risk scores—which looks at many genetic variations in a person’s DNA to predict their disease risk.

Wednesday, August 28, 2024
Leading Computational Scientist and Oncology Researcher Elana Fertig, PhD, Appointed as New Director of the Institute for Genome Sciences at the University of Maryland School of Medicine
University of Maryland School of Medicine (UMSOM) Dean Mark T. Gladwin, MD, announced today the appointment of Elana J. Fertig, PhD, FAIMBE, as the new Director of the School’s Institute for Genome Sciences (IGS). She is an internationally-recognized researcher known for her work in integrating spatial multi-omics technologies with mathematical models to develop a new predictive medicine paradigm in cancer. Spatial technologies allow researchers to learn about any cell type inside of natural tissue, including gene activity and cell interactions.

Tuesday, May 21, 2024
University of Maryland School of Medicine Launches Vaccine Development Program to Prevent Sepsis in Newborns
University of Maryland School of Medicine (UMSOM) researchers at the Center for Vaccine Development and Global Health (CVD) have been awarded up to $3.96 million over three years to develop and test a vaccine in an animal model that could eventually be used in pregnant women to prevent sepsis in newborns and infants.

Wednesday, April 05, 2023
UM School of Medicine Researchers Chart Path Forward on Developing mRNA Vaccines for Infections Beyond COVID-19
After helping to develop and test new mRNA technologies for COVID-19 vaccines, University of Maryland School of Medicine (UMSOM) researchers and scientists are turning their attention to utilizing this innovative technology to ward off other infectious diseases like malaria and influenza. Last month, UMSOM faculty in the Center for Vaccine Development and Global Health (CVD) launched a new clinical trial to investigate the use of mRNA technologies to create a vaccine against malaria. CVD Director Kathleen M. Neuzil, MD, MPH, FIDSA also provided commentary in the nation’s leading medical journal on the feasibility of using mRNA to develop a universal influenza vaccine that could eliminate the need for seasonal shots.
Thursday, December 22, 2022
A Three-Dose Malaria Vaccine Shows Safety, Efficacy in West African Adults
A three-dose regimen of a whole-parasite vaccine against malaria – called Plasmodium falciparum sporozoite (PfSPZ) vaccine – demonstrated safety and efficacy when tested in adults living in Burkina Faso, West Africa, which has endemic malaria. That is the finding of a new study published Dec. 7 in Science Translational Medicine. Researchers at the University of Maryland School of Medicine’s Center for Vaccine Development and Global Health (CVD) led the work.

Wednesday, August 24, 2022
UM School of Medicine Study Finds a New Way to Optimize Treatment Success for Fecal Transplants
Fecal transplants have been successful in treating serious diarrheal infections but have often failed when tried with other diseases. Up until now, no one could predict why these treatments sometimes failed to help restore healthy bacteria in the colon. Researchers from the University of Maryland School of Medicine’s (UMSOM) Institute for Genome Sciences (IGS) have discovered important clues that could lead more personalized approaches to optimize treatment success. They published their findings in Cell Reports Medicine online earlier this month.

Wednesday, January 26, 2022
Trial Co-led by University of Maryland School of Medicine Scientist Confirms Safety of “Mix-and-Match” COVID-19 Vaccine Booster Dosing
A University of Maryland School of Medicine (UMSOM), Center for Vaccine Development and Global Health (CVD), expert is co-leading an ongoing study that was pivotal in recommending adults and teens receive booster COVID-19 shots of their choosing starting in fall 2021. The preliminary clinical trial results, reported today in The New England Journal of Medicine, found that is safe and effective to receive boosters that are the same or a different one from the person’s primary vaccine(s).

Tuesday, November 02, 2021
Unexpected Antibody Type Found in People with Malaria Infections
Malaria, a pathogen transmitted into blood by mosquitoes in tropical climates, is typically thought of as a blood and liver infection. However, in a newly published study, researchers at the University of Maryland School of Medicine (UMSOM) have detected antibodies primarily made in response to infections in the mucous membranes — in such areas as the lungs, intestines, or vagina — in study participants with malaria.

Thursday, March 18, 2021
UM School of Medicine Helps Maryland Conduct State-Wide Sequencing of Variants in Positive COVID-19 Test Specimens
In an effort to monitor the spread of COVID-19 variants in the State of Maryland, University of Maryland School of Medicine (UMSOM) Dean E. Albert Reece, MD, PhD, MBA, announced that UMaryland Genomics at UMSOM will perform genome sequencing of variants in at least 10 percent of COVID-19 test samples, reaching an important benchmark set by the federal government to help control the spread of these variants.

Thursday, February 25, 2021
UM School of Medicine Researchers Participate in Landmark Study Detailing Sequencing of Full Human Genomes to Better Capture Genetic Diversity
Researchers at the University of Maryland School of Medicine (UMSOM) co-authored a study, published today in the journal Science, that details the sequencing of 64 full human genomes. This reference data includes individuals from around the world and better captures the genetic diversity of the human species. Among other applications, the work will enable population-specific studies on genetic predispositions to human diseases as well as the discovery of more complex forms of genetic variation.

Wednesday, February 26, 2020
Researchers Develop First Catalogue of Genes that Comprise Community of Microbes in Vaginal Microbiome
University of Maryland School of Medicine’s (UMSOM) Institute for Genome Sciences (IGS) researchers have created the first catalogue of genes that comprise the community of microbes, which inhabit the human vagina. The catalogue, called human vaginal non-redundant gene catalog (VIRGO), was recently released as a public resource that can be used by researchers to facilitate a more in-depth understanding of the role of vaginal microorganisms in women’s health and to potentially develop future treatments for certain gynecologic conditions.

Tuesday, December 03, 2019
UM School of Medicine Researchers Institute for Genome Sciences' Researchers Discover Potential New Treatment for Tropical Parasitic Disease Using Genomics
Using innovative RNA sequencing techniques, researchers at the University of Maryland School of Medicine (UMSOM) Institute for Genome Sciences identified a promising novel treatment for lymphatic filariasis, a disabling parasitic disease that is difficult to treat. The potential new therapy is an experimental cancer drug called JQ1 and targets proteins found prominently in the worm’s genome; it appears to effectively kill the adult worms in a laboratory setting, according to the study which was published today in the journal mSystems.

Wednesday, October 30, 2019
UM School of Medicine's Myron M. Levine, MD, DTPH, to Receive Prestigious Lifetime Award for Five Decades of Pioneering Vaccine Research
Myron M. Levine, MD, DTPH, the Simon and Bessie Grollman Distinguished Professor at the University of Maryland School of Medicine (UMSOM), Associate Dean for Global Health, Vaccinology and Infectious Diseases, and Founder and Former Director of the Center for Vaccine Development and Global Health (CVD) is a co-recipient of the 2020 Research! America Geoffrey Beene Foundation Builders of Science Award for his pioneering vaccine and infectious disease research.

Monday, October 21, 2019
UM School of Medicine's Kathleen M Neuzil Elected as Member of Prestigious National Academy of Medicine
Kathleen M. Neuzil, MD, MPH, Professor of Medicine and Pediatrics and Director of the Center for Vaccine Development and Global Health (CVD) at the University of Maryland School of Medicine (UMSOM), has been elected as a member of the National Academy of Medicine (NAM), in recognition of her pivotal research that has informed and shaped global vaccine and public health policy. Her membership was announced at the annual NAM meeting in Washington, D.C., placing her among the 2,178 U.S. members of this important organization. Membership in the Academy is considered one of the highest honors for individuals who have made major contributions to the advancement of the medical sciences, health care and public health.

Monday, August 19, 2019
UM School of Medicine Researcher Warns of Need for Malaria Drug to Treat Severe Cases in U.S.
Each year there are more than 200 million cases of malaria worldwide, a mosquito-borne disease caused by a parasite that brings on fever and body aches and, in some cases, more serious conditions such as coma and death. While the vast majority of these cases occur in sub-Saharan Africa and South Asia, the U.S. each year sees more than 1,500 cases, and currently there is limited access to an intravenously-administered (IV) drug needed for the more serious cases, according to a top malaria researcher at the University of Maryland School Medicine (UMSOM).
Wednesday, July 17, 2019
Vaccines Tested in UM School of Medicine’s Center for Vaccine Development Protect Children Around the World
For 30 years, the Center for Vaccine Development and Global Health (CVD) at the University of Maryland School of Medicine (UMSOM) has collaborated with the Pediatric Center of Frederick to test vaccines used in pediatric care.
Friday, March 08, 2019
UMSOM Researchers Unveil Progress and Challenges in Introducing Typhoid Conjugate Vaccine in Sub-Saharan Africa and Asia
Each year there are nearly 11 million cases of typhoid, a disease that is spread through contaminated food, drink and water. Researchers at the University of Maryland School of Medicine are leading an international consortium that is studying the impact of a typhoid conjugate vaccine (TCV) in an effort to accelerate introduction of the vaccine in countries in sub-Saharan Africa and Asia where there is a high burden of typhoid.

Wednesday, December 26, 2018
Multicenter Trial Supports Use of Topical Antibiotic as a Tool to Eliminate Staph Colonization in NICU Babies
A team of doctors led by Karen L. Kotloff, M.D., University of Maryland School of Medicine (UMSOM), Center for Vaccine Development and Global Health (CVD), has performed a clinical trial involving multiple hospitals that tested the effectiveness of applying a topical antibiotic known as mupirocin for prevention of Staphylococcus aureus (SA) infection in babies in the neonatal intensive care unit (NICU). This study was published in the journal Pediatrics.

Thursday, December 20, 2018
Data From Largest Global Diarrheal Disease Study Available to Scientists on Public Sites
Data collected from the Global Enteric Multicenter Study (GEMS), a multi-site research project studying diarrheal diseases that was designed and coordinated by researchers in the Center for Vaccine Development and Global Health (CVD) at the University of Maryland School of Medicine (UMSOM), are now available to scientists on two online data resources.
Monday, December 03, 2018
UMSOM and Groupe De Recherche Action En Sante Begin Second Typhoid Conjugate Vaccine Study in Africa
A new study has been launched in Burkina Faso for Bharat Biotech’s typhoid conjugate vaccine (TCV). It is the second clinical study underway in Africa for the vaccine and the first in West Africa. The vaccine study is a joint effort by the Center for Vaccine Development and Global Health (CVD) at the University of Maryland School of Medicine, (UMSOM) and Groupe de Recherche Action en Santé (GRAS) in Burkina Faso.

Friday, November 02, 2018
UMSOM Global Health Expert Named to Prestigious World Health Organization Immunization Panel
Kathleen Neuzil, MD, MPH, Professor of Medicine and Pediatrics and Director of the Center for Vaccine Development and Global Health (CVD) at the University of Maryland School of Medicine (UMSOM), has been named to the World Health Organization’s (WHO) Strategic Advisory Group of Experts (SAGE) on Immunization.

Sunday, October 28, 2018
American Society of Tropical Medicine & Hygiene Awards Dr. Miriam Laufer the LePrince Medal for Malaria Research
Miriam Laufer, MD, MPH, Professor of Pediatrics and Associate Director for Malaria Research at the University of Maryland School of Medicine’s (UMSOM) Center for Vaccine Development and Global Health (CVD), was awarded the Joseph Augustin LePrince Medal by the American Society of Tropical Medicine and Hygiene (ASTMH).

Thursday, May 04, 2017
UM School of Medicine Researchers Receive $9 Million Grant for Malaria Research
The University of Maryland School of Medicine has been awarded an International Center of Excellence for Malaria Research (ICEMR) grant by the National Institutes of Health’s National Institute of Allergy and Infectious Diseases (NIAID), one of seven ICEMRs awarded worldwide. With funding of more than $9 million over seven years, the grant will be used to research and develop new tools to help eliminate drug-resistant malaria in Myanmar and neighboring countries in Southeast Asia.

Friday, February 24, 2017
Experimental Malaria Vaccine Provides Durable Protection Against Multiple Strains in NIH Clinical Trial
An experimental malaria vaccine protected healthy subjects from infection with a malaria strain different from that contained in the vaccine, according to a study published this week in the Proceedings of the National Academy of Sciences (PNAS). The research was conducted by scientists at the University of Maryland School of Medicine and the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH).

Wednesday, July 06, 2016
UMSOM Begins Malaria Vaccine Trial in Burkina Faso
Malaria is one of the world’s deadliest diseases: it infects hundreds of millions of people every year, and kills about half a million, most of them under five years of age.

Wednesday, May 25, 2016
UMSOM Researchers Develop New Way to Decode Large Amounts of Biological Data
A University of Maryland School of Medicine researcher has helped develop an innovative computing technique that, on very large amounts of data, is both faster and more accurate than current methods. To spur research, a program using this technique is being offered for free to the biomedical research community.