September 08, 2022 | Vanessa McMains
University of Maryland School of Medicine Study Identifies Target for Potentially Developing New Therapies to Treat Age-Related Cognitive Decline
 One of the most upsetting aspects of age-related memory decline is not being able to remember the face that accompanies the name of a person you just talked with hours earlier. While researchers don’t understand why this dysfunction occurs, a new study conducted at University of Maryland School of Medicine (UMSOM) has provided some important new clues. The study was published on September 8 in Aging Cell.
One of the most upsetting aspects of age-related memory decline is not being able to remember the face that accompanies the name of a person you just talked with hours earlier. While researchers don’t understand why this dysfunction occurs, a new study conducted at University of Maryland School of Medicine (UMSOM) has provided some important new clues. The study was published on September 8 in Aging Cell.
Using aging mice, researchers have identified a new mechanism in neurons that causes memories associated with these social interactions to decline with age. In addition, they were able to reverse this memory loss in the lab.
The researchers report that their findings identified a specific target in the brain that may one day be used to develop therapies that could prevent or reverse memory loss due to typical aging. Aging memory problems are distinct from those caused by diseases like Alzheimer’s or dementia. At this time, there are no medications that can prevent or reverse cognitive decline due to typical aging.
“If an older adult attends a cocktail party, afterwards they would most likely recognize the names or the faces of the other attendees, but they might struggle with remembering which name went with which face,” said the study leader Michy Kelly, PhD, Associate Professor of Anatomy and Neurobiology at UMSOM.
These kinds of memories that associate multiple pieces of information within a personal interaction, so-called social associative memories, require an enzyme, known as PDE11A, in a part of the brain responsible for memory involving life experiences. Last year, Dr. Kelly published research on PDE11A demonstrating that mice with genetically similar versions of the PDE11 enzyme were more likely to interact than those mice with a different type of PDE11A. In this new study, Dr. Kelly and her team sought to determine PDE11A’s role in social associative memory in the aging brain and whether manipulating this enzyme could be used to prevent this memory loss.
Researchers can study mouse “social interactions” with their neighbors by seeing whether they will be willing to try a new food, based on their memories of encountering that food on the breath of another mouse. Mice do not like to eat new foods to avoid getting sick or even dying from it. When they smell food on another mouse’s breath, mice make an association between the food odor and the smell of the other mouse’s pheromones, the memory of which serves as a safety signal that any food with that odor is safe to eat in the future.
Dr. Kelly and her colleagues found that although old mice could recognize both food odors and social odors separately, they were not able to remember the association between the two, similar to the cognitive decline in older people.
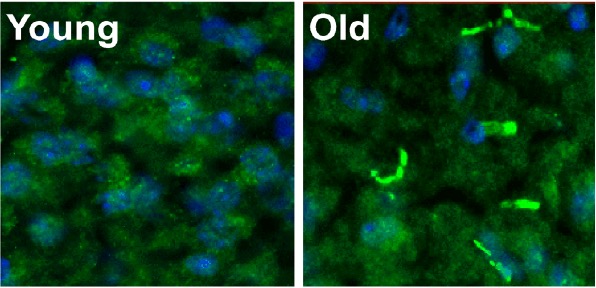
They also discovered that levels of PDE11A increased with age in both people and mice, specifically in a brain region responsible for many types of learning and memory known as the hippocampus. This extra PDE11A in the hippocampus was not simply found where it was normally located in young mice; instead, it preferentially accumulated as little filaments in compartments of neurons.
The researchers wondered if having too much PDE11A in these filaments was why the older mice forgot their social associative memories and would no longer eat the safe food they smelled on another mouse’s breath.
To answer this question, they prevented these age-related increases in PDE11A by genetically deleting the PDE11A gene in mice. Without PDE11A, the older mice no longer forgot the social associative memory, meaning they ate the safe food smelled on another mouse’s breath.
When the researchers added the PDE11A back into the hippocampus of these old mice, the mice once again forgot the social associative memory and would no longer eat the safe food.
One potential pathway to drug development to prevent this memory loss in people lies in an additional finding: The researchers learned that the concentrated filaments of PDE11A had an extra chemical modification in a specific place on the enzyme that the other PDE11 diffused throughout the neuron did not have. When they prevented this chemical modification, it reduced PDE11 levels and also prevented it from accumulating as filaments.
Dr. Kelly said, “PDE11 is involved in more things that just memory, including preferences for who you prefer to be around. So, if we are to develop a therapy to help with cognitive decline, we would not want to get rid of it entirely or it could cause other negative side effects.” She and her colleagues joke that any drug that eliminated PDE11 would ensure you would remember your friends and family, but you may no longer like them. “Thus, our goal is to figure out a way to target the bad form of PDE11A specifically, in order to not interfere with the normal, healthy function of the enzyme.”
 Mark T. Gladwin, MD, Vice President for Medical Affairs at University of Maryland, Baltimore, and the John Z. and Akiko K. Bowers Distinguished Professor and Dean at UMSOM, said: “These results suggest that the increased levels of the PDE11A enzyme that occur with age contribute to cognitive decline, and identify novel ways the enzyme might be targeted for therapeutic gain. This compelling finding warrants further investigation.”
Mark T. Gladwin, MD, Vice President for Medical Affairs at University of Maryland, Baltimore, and the John Z. and Akiko K. Bowers Distinguished Professor and Dean at UMSOM, said: “These results suggest that the increased levels of the PDE11A enzyme that occur with age contribute to cognitive decline, and identify novel ways the enzyme might be targeted for therapeutic gain. This compelling finding warrants further investigation.”
Additional authors on the study include students Nicole Gorny, MS, and Siena Petrolle of UMSOM, as well as co-authors from the University of South Carolina.
Funding for this study was provided by grants from the National Institute of General Medical Sciences (P20GM109091), the National Institute of Mental Health (R01MH101130), the National Institute on Aging (R01AG061200), and the National Science Foundation.
About the University of Maryland School of Medicine
Now in its third century, the University of Maryland School of Medicine was chartered in 1807 as the first public medical school in the United States. It continues today as one of the fastest growing, top-tier biomedical research enterprises in the world -- with 46 academic departments, centers, institutes, and programs, and a faculty of more than 3,000 physicians, scientists, and allied health professionals, including members of the National Academy of Medicine and the National Academy of Sciences, and a distinguished two-time winner of the Albert E. Lasker Award in Medical Research. With an operating budget of more than $1.3 billion, the School of Medicine works closely in partnership with the University of Maryland Medical Center and Medical System to provide research-intensive, academic, and clinically based care for nearly 2 million patients each year. The School of Medicine has nearly $600 million in extramural funding, with most of its academic departments highly ranked among all medical schools in the nation in research funding. As one of the seven professional schools that make up the University of Maryland, Baltimore campus, the School of Medicine has a total population of nearly 9,000 faculty and staff, including 2,500 students, trainees, residents, and fellows. The combined School of Medicine and Medical System (“University of Maryland Medicine”) has an annual budget of over $6 billion and an economic impact of nearly $20 billion on the state and local community. The School of Medicine, which ranks as the 8th highest among public medical schools in research productivity (according to the Association of American Medical Colleges profile) is an innovator in translational medicine, with 606 active patents and 52 start-up companies. In the latest U.S. News & World Report ranking of the Best Medical Schools, published in 2021, the UM School of Medicine is ranked #9 among the 92 public medical schools in the U.S., and in the top 15 percent (#27) of all 192 public and private U.S. medical schools. The School of Medicine works locally, nationally, and globally, with research and treatment facilities in 36 countries around the world. Visit medschool.umaryland.edu
Contact
Vanessa McMains
Director, Media & Public Affairs
University of Maryland School of Medicine
Institute of Human Virology
vmcmains@ihv.umaryland.edu
Cell: 443-875-6099
Related stories

Tuesday, August 31, 2021
Do Genetics Control Who Our Friends Are? It Seems So with Mice
Have you ever met someone you instantly liked, or at other times, someone who you knew immediately that you did not want to be friends with, although you did not know why?

Tuesday, August 31, 2021
Award-Winning Educator Adam Puche, PhD, Named Vice Chair of Department of Anatomy and Neurobiology
Asaf Keller, PhD, Professor and Chair of Anatomy and Neurobiology at the University of Maryland School of Medicine (UMSOM), along with UMSOM Dean E. Albert Reece, MD, PhD, MBA, announced that award-winning educator Adam Puche, PhD, Professor of Anatomy and Neurobiology, has been appointed to serve as the inaugural Vice Chair of that department, effective immediately.

Wednesday, November 21, 2018
UMSOM Expert Discovers Key Gene in Cells Associated with Age-Related Hearing Loss
An international group of researchers, led by Ronna Hertzano, MD, PhD, Associate Professor, Department of Otorhinolaryngology-Head & Neck Surgery, Anatomy and Neurobiology, at the University of Maryland School of Medicine (UMSOM), and Michael Bowl, Ph.D., Programme Leader Track Scientist, Mammalian Genetics Unit, MRC Harwell Institute, UK, have identified the gene that acts as a key regulator for special cells needed in hearing.