June 22, 2022 | Deborah Kotz
 Patient Survived for Two Months After First-of-Its-Kind Transplant at the University of Maryland Medical Center
Patient Survived for Two Months After First-of-Its-Kind Transplant at the University of Maryland Medical Center
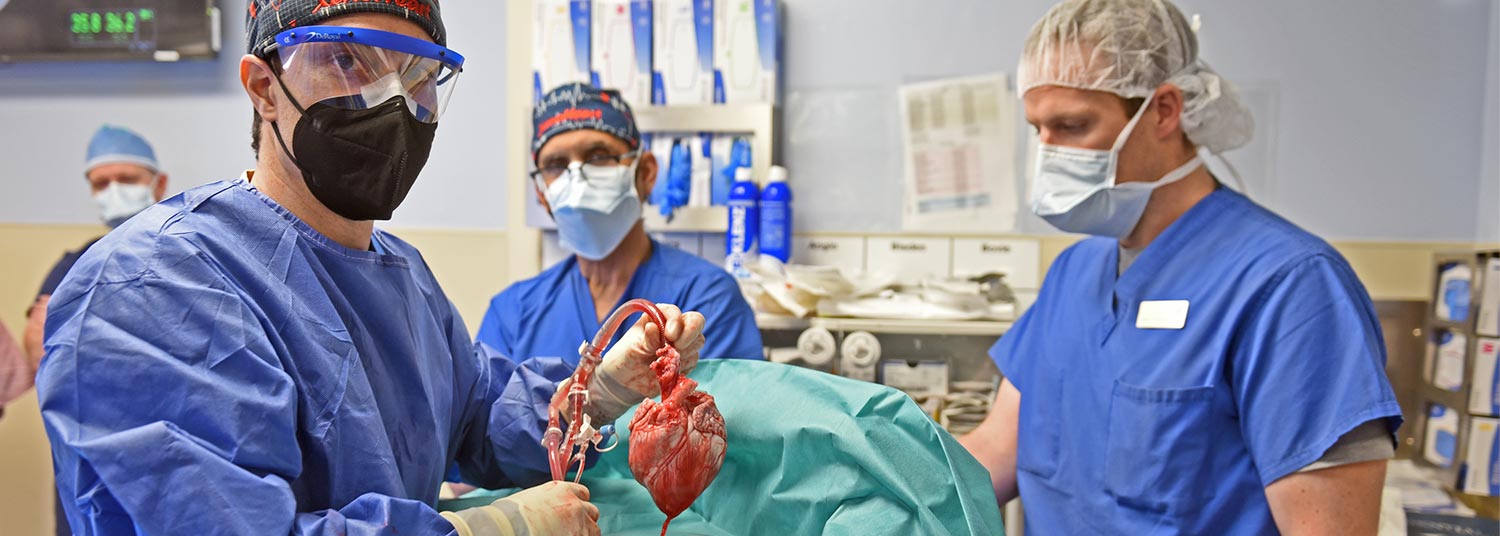
Six months ago, University of Maryland School of Medicine surgeon-scientists successfully implanted a genetically modified pig heart into a 57 year-old patient with terminal heart disease in a first-of-its-kind surgery. It was considered an early success because the patient lived for two months with a strong functioning heart showing no obvious signs of rejection, according to a new paper published today in the New England Journal of Medicine.
The historic xenotransplant surgery was conducted on January 7, 2022, by University of Maryland School of Medicine (UMSOM) faculty at the University of Maryland Medical Center (UMMC), together known as the University of Maryland Medicine. The surgery was the only available treatment option for the patient, David Bennett, who did not qualify for a traditional heart transplant and was in end-stage heart failure, nearing the end of his life. The procedure was authorized by the U.S. Food and Drug Administration under its expanded access (compassionate use) provision.
Prior to the transplant, Mr. Bennett was bedridden for eight weeks with a life-threatening arrhythmia and was connected to a heart-lung bypass machine, called extracorporeal membrane oxygenation (ECMO), to remain alive. Within days of the transplant, he was weaned from ECMO and participated in active rehabilitation for nearly two months. He visited regularly with family members and sang along to “America the Beautiful” while watching the Super Bowl in February with his physical therapist.
 “We were incredibly encouraged by his progress. His heart was strong, almost too strong for his frail body, but he had a strong will to live. He told me he wanted to go home and see his dog, Lucky,” said study co-leader Bartley Griffith, MD, Professor of Surgery and The Thomas E. and Alice Marie Hales Distinguished Professor in Transplantation at UMSOM.
“We were incredibly encouraged by his progress. His heart was strong, almost too strong for his frail body, but he had a strong will to live. He told me he wanted to go home and see his dog, Lucky,” said study co-leader Bartley Griffith, MD, Professor of Surgery and The Thomas E. and Alice Marie Hales Distinguished Professor in Transplantation at UMSOM.
The transplanted pig heart functioned well for several weeks and displayed none of the typical signs of rejection by the patient’s body, even when it was carefully examined during an autopsy, according to the analysis in the paper. The surgeons concluded that the patient died of heart failure that was likely caused by a complex array of factors.
 “We are very encouraged by this finding, and it suggests that the genetically-modified pig heart and the experimental drug we used to prevent rejection worked effectively in tandem to demonstrate that xenotransplants can potentially save future lives,” said study co-leader Muhammad M. Mohiuddin, MD, Professor of Surgery and Scientific/Program Director of the Cardiac Xenotransplantation Program at UMSOM.
“We are very encouraged by this finding, and it suggests that the genetically-modified pig heart and the experimental drug we used to prevent rejection worked effectively in tandem to demonstrate that xenotransplants can potentially save future lives,” said study co-leader Muhammad M. Mohiuddin, MD, Professor of Surgery and Scientific/Program Director of the Cardiac Xenotransplantation Program at UMSOM.
About 110,000 Americans are currently waiting for an organ transplant, and more than 6,000 patients die each year before getting one, according to the federal government’s organdonor.gov. Xenotransplantation potentially could save thousands of lives, but it does carry a unique set of risks, including the possibility of triggering a dangerous immune response that can cause the body to reject the organ.
“Our findings on autopsy did not show evidence of rejection,” said Dr. Griffith, who is also the Clinical Director of the Cardiac Xenotransplantation Program at UMSOM. “Instead, we saw a thickening and later stiffening of the heart muscle leading to diastolic heart failure, which means the heart muscle was not able to relax and fill the heart with blood as it is supposed to.”
Several factors may have contributed to the development of heart failure, including the use of intravenous immunoglobulin, IVIG, a drug that was given to the patient twice during the second month after the transplant to help prevent rejection and infection. The drug contains antibodies against pig cells that may have interacted with the pig heart, causing a reaction that damaged the heart muscle. The heart was also found to contain evidence of DNA from a latent pig virus called porcine cytomegalovirus (pCMV) through highly sensitive testing that was first detected several weeks after the surgery and was later confirmed during autopsy of the organ.
The ongoing investigations have yet to find evidence that the virus caused an infection in the patient or infected any tissues or organs beyond the heart. Whether the latent virus caused damage to the transplanted heart, contributing to the heart failure, remains under investigation.
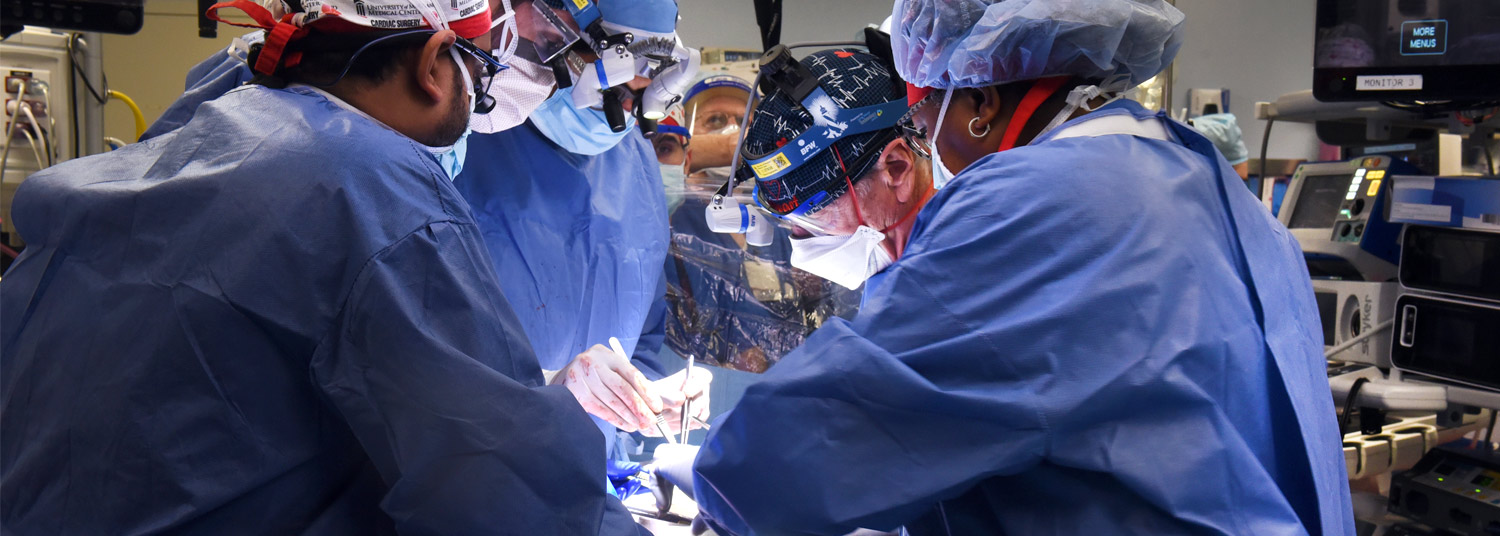
“We consider this to be an important learning experience,” Dr. Mohiuddin said. “Knowing what we know now, we will alter some of our practices and techniques in the future.”
 Before the surgery, infection control measures were carefully followed to help safeguard against known pig pathogens. The donor pig was raised in a facility using methods designed to prevent pCMV and other potential pathogens from infecting donor animals. The healthy donor pig used for the xenotransplant was screened for pathogens multiple times. It was tested just before shipment to Maryland, and just before the transplant a few days later, protocols that were accepted by the FDA.
Before the surgery, infection control measures were carefully followed to help safeguard against known pig pathogens. The donor pig was raised in a facility using methods designed to prevent pCMV and other potential pathogens from infecting donor animals. The healthy donor pig used for the xenotransplant was screened for pathogens multiple times. It was tested just before shipment to Maryland, and just before the transplant a few days later, protocols that were accepted by the FDA.
“As a prerequisite of FDA emergency authorization, we put together a hospital infection prevention plan to safeguard against the transmission of any known or unknown porcine pathogens to hospital staff and other patients,” said study co-author Kapil Saharia, MD, MPH, Assistant Professor of Medicine at the Institute of Human Virology at UMSOM. The plan included special isolation procedures, use of personal protective equipment, special handling of patient samples, and sequestration of medical instruments and equipment used for invasive procedures. “These practices remained in place for physicians and hospital staff while Mr. Bennett was treated at the University of Maryland Medical Center,” said Dr. Saharia, who is also Chief of Solid Organ Transplant Infectious Diseases Service at UMMC.
 As plans move forward for future clinical trials, more sophisticated testing techniques are being developed and validated to ensure that this virus or any other does not go undetected.
As plans move forward for future clinical trials, more sophisticated testing techniques are being developed and validated to ensure that this virus or any other does not go undetected.
“We were pioneers with this first-in-the-world surgery, and we have learned so much from this experience,” said study co-author Christine Lau, MD, MBA, the Dr. Robert W. Buxton Professor, Chair of the Department of Surgery at UMSOM, and Surgeon-in-Chief at UMMC. “I think we have moved the field of transplantation one important step closer to making this a clinical reality for patients in need.”
“This publication will provide vital information for the xenotransplant research community and will play a pivotal role in pushing this field forward,” said E. Albert Reece, MD, PhD, MBA, Executive Vice President for Medical Affairs, UM Baltimore, and the John Z. and Akiko K. Bowers Distinguished Professor and Dean, University of Maryland School of Medicine. "I am so proud of the historic medical achievements made by these leaders in their field and hope these first brave steps, including the courage of Mr. Bennett, will lead to a long-term solution where no patient will die waiting for an organ transplant.”
“I applaud the many years of complex research and steadfast commitment behind this extraordinary scientific achievement,” said Bert W. O'Malley, MD, President and CEO, University of Maryland Medical Center. “We have entered a new era in organ transplantation. While we still have a road ahead before xenotransplantation becomes an everyday reality, this historic surgery brings a future many never thought possible within our reach.”
Additional UMSOM faculty co-authors on the paper include:
* Corbin Goerlich, MD, Surgical Resident and Doctoral Fellow
* Avneesh Singh, PhD, Immunologist and Assistant Professor in Department of Surgery
* Aakash Shah, MD, Cardiac Surgery Resident in the Department of Surgery
* Alison Grazioli, MD, Assistant Professor of Medicine and Medical Director of the Cardiac Surgery Intensive Care Unit at UMMC
* Susie Hong, MD, Cardiologist, Assistant Professor of Medicine
* Susan Joseph, MD, Cardiologist, Associate Professor of Medicine and Section Chief, Advanced Heart Failure and Transplant Team at UMMC
“This peer-reviewed publication represents the culmination of more than two decades of work by Revivicor scientists and provides the deepest insight yet into how xenotransplantation can successfully address the critical shortage of tolerable, transplantable organs,” said study co-author David Ayares, PhD, President and Chief Scientific Officer of Revivicor, Inc. UMSOM received a $15.7 million sponsored research grant to evaluate Revivicor genetically modified pig UHearts™ in baboon studies. “The learnings from this procedure, now available to the global scientific community because of the selfless gift of Mr. Bennett and his family, bring us one step closer to saving countless lives in the future.”
About the University of Maryland School of Medicine
Now in its third century, the University of Maryland School of Medicine was chartered in 1807 as the first public medical school in the United States. It continues today as one of the fastest growing, top-tier biomedical research enterprises in the world -- with 46 academic departments, centers, institutes, and programs, and a faculty of more than 3,000 physicians, scientists, and allied health professionals, including members of the National Academy of Medicine and the National Academy of Sciences, and a distinguished two-time winner of the Albert E. Lasker Award in Medical Research. With an operating budget of more than $1.3 billion, the School of Medicine works closely in partnership with the University of Maryland Medical Center and Medical System to provide research-intensive, academic and clinically based care for nearly 2 million patients each year. The School of Medicine has nearly $600 million in extramural funding, with most of its academic departments highly ranked among all medical schools in the nation in research funding. As one of the seven professional schools that make up the University of Maryland, Baltimore campus, the School of Medicine has a total population of nearly 9,000 faculty and staff, including 2,500 students, trainees, residents, and fellows. The combined School of Medicine and Medical System (“University of Maryland Medicine”) has an annual budget of over $6 billion and an economic impact of nearly $20 billion on the state and local community. The School of Medicine, which ranks as the 8th highest among public medical schools in research productivity (according to the Association of American Medical Colleges profile) is an innovator in translational medicine, with 606 active patents and 52 start-up companies. In the latest U.S. News & World Report ranking of the Best Medical Schools, published in 2021, the UM School of Medicine is ranked #9 among the 92 public medical schools in the U.S., and in the top 15 percent (#27) of all 192 public and private U.S. medical schools. The School of Medicine works locally, nationally, and globally, with research and treatment facilities in 36 countries around the world. Visit medschool.umaryland.edu
About the University of Maryland Medical Center
The University of Maryland Medical Center (UMMC) is comprised of two hospital campuses in Baltimore: the 800-bed flagship institution of the 12-hospital University of Maryland Medical System (UMMS) and the 200-bed UMMC Midtown Campus. Both campuses are academic medical centers for training physicians and health professionals and for pursuing research and innovation to improve health. UMMC's downtown campus is a national and regional referral center for trauma, cancer care, neurosciences, advanced cardiovascular care, and women's and children's health, and has one of the largest solid organ transplant programs in the country. All physicians on staff at the downtown campus are clinical faculty physicians of the University of Maryland School of Medicine. The UMMC Midtown Campus medical staff is predominantly faculty physicians specializing in a wide spectrum of medical and surgical subspecialties, primary care for adults and children and behavioral health. UMMC Midtown has been a teaching hospital for 140 years and is located one mile away from the downtown campus. For more information, visit www.umm.edu.
About the University of Maryland Medical System
The University of Maryland Medical System (UMMS) is a university-based regional health care system focused on serving the health care needs of Maryland, bringing innovation, discovery and research to the care we provide and educating the state’s future physician and health care professionals through our partnership with the University of Maryland School of Medicine and University of Maryland, Baltimore professional schools (Nursing, Pharmacy, Social Work and Dentistry) in Baltimore. As one of the largest private employers in the State, the health system’s more than 29,500 employees and 4,000 affiliated physicians provide primary and specialty care in more than 150 locations, including 12 hospitals and 10 University of Maryland Urgent Care centers. The UMMS flagship academic campus, the University of Maryland Medical Center in downtown Baltimore, is recognized regionally and nationally for excellence and innovation in specialized care. Our acute care and specialty rehabilitation hospitals serve urban, suburban and rural communities and are located in 13 counties across the State. For more information, visit www.umms.org.
UHeart is a trademark of Lung Biotechnology PBC.
Mohiuddin is listed on a patent application filed in connection with this procedure.
Resources for the Media:
Contact
Deborah Kotz
Senior Director of Media Relations
Office of Public Affairs & Communications
University of Maryland School of Medicine
Email: DKotz@som.umaryland.edu
o: 410-706-4255
c: 410-804-0054
t: @debkotz2
Related stories
Wednesday, January 08, 2025
Presenting a Path Forward for Future Genetically-Modified Pig Heart Transplants: Lessons Learned from Second Patient
Continuing significant advancements in the field of xenotransplantation, surgeon-scientists from the University of Maryland School of Medicine provided an extensive analysis on the second patient in the world to receive a genetically-modified pig organ. Lawrence Faucette, 58, received a pig heart at the University of Maryland Medical Center in 2023 to treat his end-stage heart failure. He lived for 40 days before choosing to forgo additional treatment after the transplant began to fail due to rejection.

Wednesday, January 31, 2024
Renowned Surgeon Dr. Bartley Griffith Named Vice Chair for Innovation in UM School of Medicine’s Department of Surgery
University of Maryland School of Medicine (UMSOM) Department of Surgery Chair Christine Lau, MD, MBA, along with UMSOM Dean Mark T. Gladwin, MD, announced today the appointment of Bartley P. Griffith, MD, as the Department of Surgery’s first Vice Chair for Innovation. In this role, Dr. Griffith will nurture a culture of innovation, entrepreneurship, and collaboration in the Department, and expand the integration of related sciences into surgical practice. The appointment is effective on February 1.
Tuesday, October 31, 2023
In Memoriam: Lawrence Faucette
It is with great sadness that we announce the passing of Lawrence Faucette, the 58-year-old patient with terminal heart disease who received the world’s second genetically-modified pig heart transplant. Mr. Faucette received the transplant on September 20 and lived for nearly six weeks following the surgery.

Friday, September 22, 2023
UM Medicine Faculty-Scientists and Clinicians Perform Second Historic Transplant of Pig Heart into Patient with End-Stage Cardiovascular Disease
A 58-year-old patient with terminal heart disease became the second patient in the world to receive a historic transplant of a genetically-modified pig heart on September 20. He is recovering and communicating with his loved ones. This is only the second time in the world that a genetically modified pig heart has been transplanted into a living patient. Both historic surgeries were performed by University of Maryland School of Medicine (UMSOM) faculty at the University of Maryland Medical Center (UMMC).
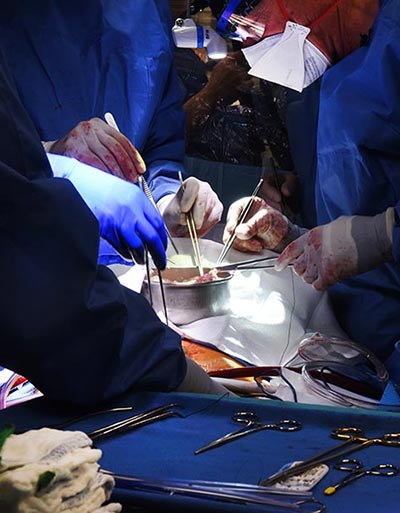
Friday, June 30, 2023
Lessons Learned from World’s First Successful Transplant of Genetically-Modified Pig Heart into Human Patient
A new study published today in the Lancet has revealed the most extensive analysis to date on what led to the eventual heart failure in the world's first successful transplant of a genetically-modified pig heart into a human patient. This groundbreaking procedure was conducted by University of Maryland School of Medicine (UMSOM) physician-scientists back in January 2022 and marked an important milestone for medical science.

Friday, December 16, 2022
UM School of Medicine Surgeon-Scientist Named One of Nature’s 10 People Who Helped Shape the Science Stories of 2022
The world-renowned journal Nature, named Muhammad Mohiuddin, MD, DSc, Program and Scientific Director of the Cardiac Xenotransplantation Program at the University of Maryland School of Medicine (UMSOM), on its annual list of 10 people who helped shaped science in 2022. His pivotal work over the past three decades transplanting genetically-modified pig hearts into non-human primates led to the historic xenotransplant of a pig heart into a human patient this past January. The surgery was led by Bartley Griffith, MD, the Thomas E. and Alice Marie Hales Distinguished Professor of Transplant Surgery and Clinical Director of the Cardiac Xenotransplantation Program, who was also recognized by Nature for his ground-breaking efforts to move the field of transplantation into a new era.

Monday, November 14, 2022
Unexpected Electrical Changes Seen in First Successful Transplant of Genetically-Modified Pig Heart
Ten months after transplanting the first genetically-modified pig heart into a human patient, University of Maryland School of Medicine (UMSOM) researchers continue to report on new findings from the landmark transplant. Their latest study demonstrates for the first time that unexpected electrical changes occurred in the pig heart transplanted into the patient David Bennett. The findings were presented at the American Heart Association (AHA) meeting this past weekend.