December 03, 2018 | Joanne Morrison
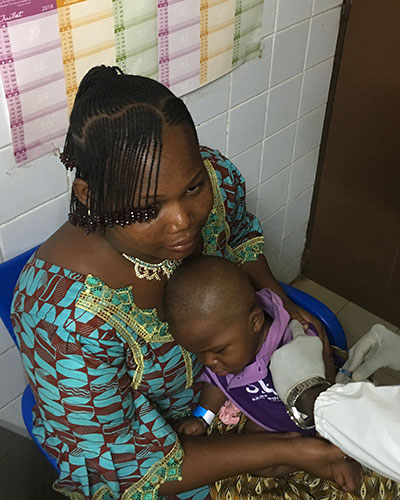
Scientists to Enroll 250 Participants in Burkina Faso Typhoid Conjugate Vaccine Study
A new study has been launched in Burkina Faso for Bharat Biotech’s typhoid conjugate vaccine (TCV). It is the second clinical study underway in Africa for the vaccine and the first in West Africa. The vaccine study is a joint effort by the Center for Vaccine Development and Global Health (CVD) at the University of Maryland School of Medicine, (UMSOM) and Groupe de Recherche Action en Santé (GRAS) in Burkina Faso.
Typhoid, a serious enteric fever caused by Salmonella Typhi, is spread through contaminated food and water. While largely eliminated in industrialized countries, typhoid continues to be a substantial public health issue that disproportionately impacts children and marginalized populations in much of Asia, sub-Saharan Africa, and parts of Latin America and the Middle East.
The study is part of the Typhoid Vaccine Acceleration Consortium (TyVAC), an international consortium working to advance the introduction of TCVs in typhoid-endemic regions. This vaccine is considered to be the most effective in protecting against typhoid because it provides longer-lasting protection and can be administered to children under two years of age, allowing for inclusion in routine immunization programs.
While this new typhoid vaccine has recently been recommended by the World Health Organization (WHO) there are no data on co-administration of the TCV with other routine vaccines. Researchers will study the efficacy in Burkinabe children and how their immune systems respond to the vaccine when given with other vaccines, such as yellow fever and meningitis A vaccines.
 “This is an important collaboration with our partners in Burkina Faso to test this vaccine in the West African setting,” said Kathleen Neuzil, MD, MPH, UMSOM Professor of Medicine and Pediatrics and Director of the CVD and TyVAC Principal Investigator. “Millions of children are set to be better protected from typhoid fever through the broader global efforts to accelerate the use of the vaccine in conjunction with improving water, sanitation and hygiene.”
“This is an important collaboration with our partners in Burkina Faso to test this vaccine in the West African setting,” said Kathleen Neuzil, MD, MPH, UMSOM Professor of Medicine and Pediatrics and Director of the CVD and TyVAC Principal Investigator. “Millions of children are set to be better protected from typhoid fever through the broader global efforts to accelerate the use of the vaccine in conjunction with improving water, sanitation and hygiene.”
The Burkina Faso study comes after the WHO pre-qualified the vaccine in 2017 and as GAVI, the Vaccine Alliance, committed U.S. $85 million in funding to help introduce a TCV typhoid-endemic countries.
“We are very enthusiastic about this opportunity to study the TCV and anticipate that the data from this study will inform decision-makers in the region highly affected by typhoid,” said Sodiomon B Sirima, MD, PhD, the Scientific Director of GRAS and Co-Principal Investigator of the study onsite in Ouagadougou.
The burden of typhoid is likely underestimated due to difficulties in surveillance and diagnostic challenges, but current estimates indicate that each year there are nearly 12 million cases and more than 128,000 deaths, with young children and adolescents aged 2 to 15 years disproportionately impacted. Though treatable with antibiotics, the rate of cases resistant to the available antibiotics is increasing. Vaccination and improvements in water, sanitation, and hygiene are key components of an integrated strategy to prevent typhoid.
About the Burkina Faso Study
 Matthew Laurens, MD, MPH, Associate Professor of Pediatrics at UMSOM is a co- investigator in the trial, which will include 250 volunteers aged nine months to two years old. The investigators plan to vaccinate 100 children between the ages of 9-11 months, and 150 children between the ages of 15 months and 2 years, in Ouagadougou, Burkina Faso, with either TCV or the polio vaccine.
Matthew Laurens, MD, MPH, Associate Professor of Pediatrics at UMSOM is a co- investigator in the trial, which will include 250 volunteers aged nine months to two years old. The investigators plan to vaccinate 100 children between the ages of 9-11 months, and 150 children between the ages of 15 months and 2 years, in Ouagadougou, Burkina Faso, with either TCV or the polio vaccine.
The children will then have follow-up visits on days 3, 7, 28 and 180. Data from the study are expected to help inform policymakers about implementing a mass TCV campaign in Burkina Faso, which was the very first country to conduct a successful mass vaccination campaign against meningitis A.
Burkina Faso has a well-established research infrastructure, an ongoing typhoid surveillance program, and a history of early introduction of WHO pre-qualified vaccines. From 2011 to 2014, Burkina Faso was part of the International Vaccine Institute Typhoid Surveillance in Africa Program (TSAP), which showed a significant burden of typhoid in the country.
Testing the vaccine in West Africa is important given differences in population characteristics such as malnutrition and HIV prevalence, as well as the epidemiology and differing strains, attack rates and severity of typhoid.
About TyVAC
The Typhoid Vaccine Acceleration Consortium (TyVAC), is led by UMSOM’s CVD, the Oxford Vaccine Group at the University of Oxford, and PATH. It is funded by the Bill & Melinda Gates Foundation.
TyVAC works closely with local and global partners to accelerate the introduction of TCVs in low-income countries and facilitate access to typhoid vaccines in the most at-risk and marginalized communities. Using a multidisciplinary approach—at the global level, TyVAC works closely with WHO, Gavi, and other stakeholders to ensure there are sufficient data and evidence to inform global guidelines, financing decisions, and a sustainable vaccine supply. Similarly, TyVAC works with local partners to support program preparation and ensure evidence-based policy decisions. TyVAC assesses existing data and generates new evidence related to typhoid disease burden, antimicrobial resistance, cost-effectiveness, health impact analyses, and regional data on TCVs. Country-level analyses help quantify the cost and economic value of vaccines and inform decision makers at the national level. TyVAC is committed to ensuring that prevention and control of typhoid is a global health priority. TyVAC is working with partners to take an integrated approach that includes TCVs with improved water, sanitation, and hygiene, to mitigate the substantial and detrimental impact of typhoid.
About the University of Maryland School of Medicine
Now in its third century, the University of Maryland School of Medicine was chartered in 1807 as the first public medical school in the United States. It continues today as one of the fastest growing, top-tier biomedical research enterprises in the world -- with 43 academic departments, centers, institutes, and programs; and a faculty of more than 3,000 physicians, scientists, and allied health professionals, including members of the National Academy of Medicine and the National Academy of Sciences, and a distinguished recipient of the Albert E. Lasker Award in Medical Research. With an operating budget of more than $1 billion, the School of Medicine works closely in partnership with the University of Maryland Medical Center and Medical System to provide research-intensive, academic and clinically-based care for more than 1.2 million patients each year. The School has over 2,500 students, residents, and fellows, and more than $530 million in extramural funding, with most of its academic departments highly ranked among all medical schools in the nation in research funding. As one of the seven professional schools that make up the University of Maryland, Baltimore campus, the School of Medicine has a total workforce of nearly 7,000 individuals. The combined School and Medical System (“University of Maryland Medicine”) has an annual budget of nearly $6 billion and an economic impact more than $15 billion on the state and local community. The School of Medicine faculty, which ranks as the 8thhighest among public medical schools in research productivity, is an innovator in translational medicine, with 600 active patents and 24 start-up companies. The School works locally, nationally, and globally, with research and treatment facilities in 36 countries around the world. Visit medschool.umaryland.edu/
About the UMSOM Center for Vaccine Development and Global Health
For over 40 years, researchers in the Center for Vaccine Development and Global Health have worked domestically and internationally to develop, test, and deploy vaccines to aid the world’s underserved populations. CVD is an academic enterprise engaged in the full range of infectious disease intervention from basic laboratory research through vaccine development, pre-clinical and clinical evaluation, large-scale pre-licensure field studies, and post-licensure assessments. CVD has worked to eliminate vaccine-preventable diseases. CVD has created and tested vaccines against cholera, typhoid fever, paratyphoid fever, non-typhoidal salmonella disease, shigellosis (bacillary dysentery), Escherichia coli diarrhea, nosocomial pathogens, tularemia, influenza, and other infectious diseases.
CVD’s research covers the broader goal of improving global health by conducting innovative, leading research in Baltimore and around the world. CVD researchers are developing new and improved ways to diagnose, prevent, treat, control, and eradicate diseases of global impact. Currently, these diseases include malaria, typhoid, shigella and vaccine-preventable infectious diseases. CVD researchers have been involved in critical vaccine development for emerging pathogens such as Zika and Ebola. In addition, CVD’s work focuses on the ever-growing challenge of anti-microbial resistance.
About Groupe de Recherche Action en Santé (GRAS)
GRAS is a medical research institute existing under the laws of Burkina Faso, operating in the field of human health since 2008 with a focus on clinical research. Its main offices are located in Ouagadougou, the capital of Burkina Faso and its main missions are, among others, to design and carry out clinical research and operational and fundamental studies to identify new tools for the control of target diseases and monitoring the effectiveness of the existing one; to perform the design, the implementation and the monitoring-evaluation of health programs and projects; and to strengthen professional skills through training and assistance of teams in the conduct of clinical research.
The scientific staff of GRAS is multidisciplinary with well-proven skills in clinical trials. The staff has a sound knowledge of ethical principles in research involving human subjects and on ICH GCP. Laboratory staff are certified for the shipment of dangerous goods. The Data Manager has strong experience with several data management software programs. IT features a real-time management system for clinical trials data with a system network and an internet broadband access, and a data archiving system according to GCP. GRAS has a well-established cold chain. The main clinical trial unit of GRAS is embedded in a tertiary hospital located in Ouagadougou which provide out and in patients cares, maternal and child health services and emergency services. The hospital also provides services such ultrasound, X-ray and magnetic resonance imaging and has a medical laboratory well-equipped engaged in an external quality control program as part of its commitments to quality. The trial unit space is organized to ensure that all the key steps in the path of clinical trial participant journey have a dedicated space (consenting rooms, clinical examination rooms, biological sampling room, IP preparation and administration rooms post vaccination surveillance room well-equipped for resuscitation). GRAS has access to the Ouagadougou Health and Demographic Surveillance System (HDSS) which is a real asset for the conduct of epidemiological and clinical trials. It covers about 90,000 inhabitants, established in 2008 in five neighborhoods (two formal and three informal settlements or slums) of Ouagadougou, located roughly at 2 kilometers of the clinical unit of GRAS. During the last decade, scientists from GRAS in collaboration with its national and international partners have carried out several epidemiological studies, health system research, drugs and vaccines trials from phase 1 to phase 4 and for various diseases such as malaria, pneumonia, sickle cell diseases, and hepatitis.
Contact
Office of Public Affairs
655 West Baltimore Street
Bressler Research Building 14-002
Baltimore, Maryland 21201-1559
Contact Media Relations
(410) 706-5260
Related stories

Tuesday, May 21, 2024
University of Maryland School of Medicine Launches Vaccine Development Program to Prevent Sepsis in Newborns
University of Maryland School of Medicine (UMSOM) researchers at the Center for Vaccine Development and Global Health (CVD) have been awarded up to $3.96 million over three years to develop and test a vaccine in an animal model that could eventually be used in pregnant women to prevent sepsis in newborns and infants.

Wednesday, April 05, 2023
UM School of Medicine Researchers Chart Path Forward on Developing mRNA Vaccines for Infections Beyond COVID-19
After helping to develop and test new mRNA technologies for COVID-19 vaccines, University of Maryland School of Medicine (UMSOM) researchers and scientists are turning their attention to utilizing this innovative technology to ward off other infectious diseases like malaria and influenza. Last month, UMSOM faculty in the Center for Vaccine Development and Global Health (CVD) launched a new clinical trial to investigate the use of mRNA technologies to create a vaccine against malaria. CVD Director Kathleen M. Neuzil, MD, MPH, FIDSA also provided commentary in the nation’s leading medical journal on the feasibility of using mRNA to develop a universal influenza vaccine that could eliminate the need for seasonal shots.
Monday, July 18, 2022
New Genomic Research Shows Why Testing Malaria Vaccines in the Clinic is as Rigorous as Natural Exposure in the Field
Malaria is the deadliest mosquito-borne parasitic infection of humans. In 2021, after a century of research, the World Health Organization (WHO) approved the world’s first malaria vaccine. That vaccine reduces the incidence of malaria infections in young children aged 5-17 months by only 30 percent, meaning that it remains critical to continue developing and testing more effective vaccines.

Wednesday, January 26, 2022
Trial Co-led by University of Maryland School of Medicine Scientist Confirms Safety of “Mix-and-Match” COVID-19 Vaccine Booster Dosing
A University of Maryland School of Medicine (UMSOM), Center for Vaccine Development and Global Health (CVD), expert is co-leading an ongoing study that was pivotal in recommending adults and teens receive booster COVID-19 shots of their choosing starting in fall 2021. The preliminary clinical trial results, reported today in The New England Journal of Medicine, found that is safe and effective to receive boosters that are the same or a different one from the person’s primary vaccine(s).

Thursday, July 02, 2020
UMSOM Researchers Help Weigh Role of Human Challenge Studies for COVID-19 Vaccine Development
Members of the National Institutes of Health’s (NIH) Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) Working Group, which includes Kathleen Neuzil, MD, MPH, DTPH, the Myron M. Levine, MD, DTPH Professor in Vaccinology and Director of the Center for Vaccine Development and Global Health (CVD) at the University of Maryland School of Medicine (UMSOM), assessed the practical considerations and prerequisites for using controlled human infection models (CHIMs) to support development of a SARS-CoV-2 Vaccine.

Wednesday, October 30, 2019
UM School of Medicine's Myron M. Levine, MD, DTPH, to Receive Prestigious Lifetime Award for Five Decades of Pioneering Vaccine Research
Myron M. Levine, MD, DTPH, the Simon and Bessie Grollman Distinguished Professor at the University of Maryland School of Medicine (UMSOM), Associate Dean for Global Health, Vaccinology and Infectious Diseases, and Founder and Former Director of the Center for Vaccine Development and Global Health (CVD) is a co-recipient of the 2020 Research! America Geoffrey Beene Foundation Builders of Science Award for his pioneering vaccine and infectious disease research.

Monday, October 21, 2019
UM School of Medicine's Kathleen M Neuzil Elected as Member of Prestigious National Academy of Medicine
Kathleen M. Neuzil, MD, MPH, Professor of Medicine and Pediatrics and Director of the Center for Vaccine Development and Global Health (CVD) at the University of Maryland School of Medicine (UMSOM), has been elected as a member of the National Academy of Medicine (NAM), in recognition of her pivotal research that has informed and shaped global vaccine and public health policy. Her membership was announced at the annual NAM meeting in Washington, D.C., placing her among the 2,178 U.S. members of this important organization. Membership in the Academy is considered one of the highest honors for individuals who have made major contributions to the advancement of the medical sciences, health care and public health.

Friday, September 20, 2019
UM School of Medicine's Center for Vaccine Development and Global Health Receives NIH Contract of up to More than $200 Million for Influenza Research
Kathleen Neuzil, MD, MPH, Professor of Medicine and Pediatrics and Director of the Center for Vaccine Development and Global Health (CVD) at the University of Maryland School of Medicine (UMSOM) and Dean E. Albert Reece, MD, PhD, MBA, announced that CVD has been awarded a contract from the National Institute of Allergy and Infectious Diseases (NIAID), with total funding up to more than $200 million over seven years if all contract options are exercised.

Monday, August 19, 2019
UM School of Medicine Researcher Warns of Need for Malaria Drug to Treat Severe Cases in U.S.
Each year there are more than 200 million cases of malaria worldwide, a mosquito-borne disease caused by a parasite that brings on fever and body aches and, in some cases, more serious conditions such as coma and death. While the vast majority of these cases occur in sub-Saharan Africa and South Asia, the U.S. each year sees more than 1,500 cases, and currently there is limited access to an intravenously-administered (IV) drug needed for the more serious cases, according to a top malaria researcher at the University of Maryland School Medicine (UMSOM).

Tuesday, August 13, 2019
UM School of Medicine Researchers Begin Phase 1 Clinical Trial of Vaccine Against Mosquito-Borne Diseases
Mosquito-borne diseases including malaria, dengue and yellow fever, have a severe impact resulting in millions of deaths worldwide, hitting the world’s most vulnerable populations the hardest. Researchers at the University of Maryland School of Medicine (UMSOM) have begun testing an experimental vaccine that is designed to protect against a series of these diseases.
Wednesday, July 17, 2019
Vaccines Tested in UM School of Medicine’s Center for Vaccine Development Protect Children Around the World
For 30 years, the Center for Vaccine Development and Global Health (CVD) at the University of Maryland School of Medicine (UMSOM) has collaborated with the Pediatric Center of Frederick to test vaccines used in pediatric care.
Friday, March 08, 2019
UMSOM Researchers Unveil Progress and Challenges in Introducing Typhoid Conjugate Vaccine in Sub-Saharan Africa and Asia
Each year there are nearly 11 million cases of typhoid, a disease that is spread through contaminated food, drink and water. Researchers at the University of Maryland School of Medicine are leading an international consortium that is studying the impact of a typhoid conjugate vaccine (TCV) in an effort to accelerate introduction of the vaccine in countries in sub-Saharan Africa and Asia where there is a high burden of typhoid.

Wednesday, December 26, 2018
Multicenter Trial Supports Use of Topical Antibiotic as a Tool to Eliminate Staph Colonization in NICU Babies
A team of doctors led by Karen L. Kotloff, M.D., University of Maryland School of Medicine (UMSOM), Center for Vaccine Development and Global Health (CVD), has performed a clinical trial involving multiple hospitals that tested the effectiveness of applying a topical antibiotic known as mupirocin for prevention of Staphylococcus aureus (SA) infection in babies in the neonatal intensive care unit (NICU). This study was published in the journal Pediatrics.

Thursday, December 20, 2018
Data From Largest Global Diarrheal Disease Study Available to Scientists on Public Sites
Data collected from the Global Enteric Multicenter Study (GEMS), a multi-site research project studying diarrheal diseases that was designed and coordinated by researchers in the Center for Vaccine Development and Global Health (CVD) at the University of Maryland School of Medicine (UMSOM), are now available to scientists on two online data resources.

Friday, November 02, 2018
UMSOM Global Health Expert Named to Prestigious World Health Organization Immunization Panel
Kathleen Neuzil, MD, MPH, Professor of Medicine and Pediatrics and Director of the Center for Vaccine Development and Global Health (CVD) at the University of Maryland School of Medicine (UMSOM), has been named to the World Health Organization’s (WHO) Strategic Advisory Group of Experts (SAGE) on Immunization.

Sunday, October 28, 2018
American Society of Tropical Medicine & Hygiene Awards Dr. Miriam Laufer the LePrince Medal for Malaria Research
Miriam Laufer, MD, MPH, Professor of Pediatrics and Associate Director for Malaria Research at the University of Maryland School of Medicine’s (UMSOM) Center for Vaccine Development and Global Health (CVD), was awarded the Joseph Augustin LePrince Medal by the American Society of Tropical Medicine and Hygiene (ASTMH).

Monday, April 23, 2018
UM School of Medicine Launches Newly Organized Center for Vaccine Development and Global Health Under Leadership of International Vaccine Expert Dr. Kathleen Neuzil
University of Maryland School of Medicine (UMSOM) Dean E. Albert Reece, MD, PhD, MBA, announced today the launch of a newly organized Center for Vaccine Development and Global Health (CVD). The new Center will be led by UMSOM Professor of Medicine and Pediatrics, Kathleen Neuzil, MD MPH, FIDSA, one of the world’s most influential research scientists and advocates in vaccine development and policy.

Thursday, September 28, 2017
UM SOM Vaccine Expert Warns of Risks of Influenza to Older Populations
“Protect yourself and others by getting the flu shot” was the message from Kathleen Neuzil, MD, MPH, FIDSA, Director of the Center for Vaccine Development (CVD) at the University of Maryland School of Medicine (UM SOM). Dr. Neuzil was among the speakers at a press conference hosted by the National Foundation for Infectious Diseases. U.S. Secretary of Health and Human Services, Thomas E Price, MD, also spoke at the press conference and urged people to get their flu shot. The panel members all received their flu shots following the press conference.
Monday, May 15, 2017
U.S. CDC Recommends Use of Cholera Vaccine Developed by University of Maryland School of Medicine
A cholera vaccine developed by scientists at the University of Maryland School of Medicine’s Center for Vaccine Development (CVD) has been recommended by the U.S. Centers for Disease Control and Prevention (CDC) for use as a protection for U.S. adults traveling to areas with cholera. The CDC’s latest recommendation was published on May 11, in Morbidity and Mortality Weekly Report.

Thursday, November 10, 2016
International Consortium Receives $36.9 Million Grant to Fight Typhoid
Typhoid fever, a bacterial infection that causes high fever and other disabling symptoms, remains a serious global problem in the developing world: it kills almost a quarter of a million people annually, and infects about 21 million.

Thursday, September 22, 2016
University of Maryland School of Medicine Holds Inaugural Global Health Summit
The Institute for Global Health (IGH) at the University of Maryland School of Medicine (UM SOM) will hold its first Global Health Summit on September 26. The event, which will take place from noon to 6 p.m. in the SMC Campus Center at 621 W. Lombard Street, will seek to foster collaboration among scientists and promote new and innovative global health research at UM SOM, as well as at other schools at the University of Maryland, Baltimore (UMB). Faculty and staff from UM SOM, as well as other schools are welcome at the event.
Thursday, August 04, 2016
UM SOM is Center Stage in Testing National Institutes of Health Zika Vaccine
UM SOM'S Center for Vaccine Development, tapped previously for Ebola vaccine, now steps up to help develop first Zika vaccine.

Wednesday, July 06, 2016
UMSOM Begins Malaria Vaccine Trial in Burkina Faso
Malaria is one of the world’s deadliest diseases: it infects hundreds of millions of people every year, and kills about half a million, most of them under five years of age.

Wednesday, June 29, 2016
UM SOM Researchers Awarded Grant to Use Innovative Alternative to Autopsies to Better Understand Child Mortality
Kathleen Neuzil, MD, MPH, director of the Center for Vaccine Development (CVD) at the University of Maryland School of Medicine (UM SOM), and UM SOM Dean E. Albert Reece, MD, PhD, MBA, announced today that CVD has been awarded a large grant from the Bill & Melinda Gates Foundation for research that will help determine why so many children under five are dying in the world’s poorest countries. The grant will fund use of an innovative alternative to traditional autopsy known as minimally invasive tissue sampling. The technique, which involves the collection of tissue samples with fine needles, allows researchers to quickly identify the cause of death, and help illuminate ways to save lives and improve the health of children in these vulnerable areas.

Tuesday, April 12, 2016
UM SOM Researcher Dr. Kathleen Neuzil Honored by Vanderbilt University School of Medicine
The Vanderbilt University School of Medicine (VUSM) has announced that Kathleen Neuzil, MD, MPH, director of the Center for Vaccine Development at the University of Maryland School of Medicine (UM SOM), has received the 2016 Vanderbilt University School of Medicine Distinguished Alumni Award.
