Cellular crosstalk through the release of extracellular vesicles (EVs) is a rapidly emerging concept in biology and medicine. All cells release these nanoscale messengers with biological cargo that can modulate the function of recipient cells. EVs can be formed directly at the plasma membrane (size range: <50-1000nm) or within an endosome (size range: <50-200nm; canonically termed “exosomes”) before being released into the extracellular space. In fact, EVs can be isolated from most biological fluids, sparking much interest in their use as clinical biomarkers. The research community continues to actively refine the classification of EV subtypes as we gain a greater appreciation and understanding of their diversity (see Théry, Witwer et al., 2018; Jeppesen et al., 2019).
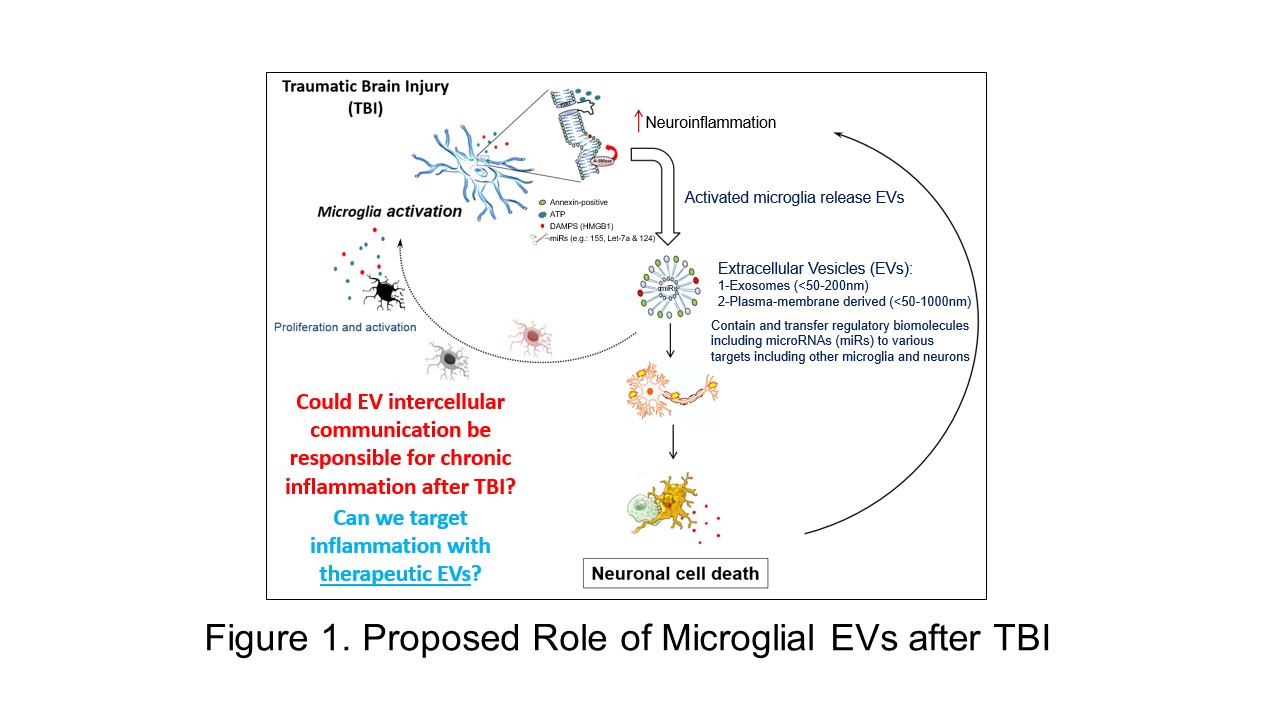
A major research interest in the lab is to understand the role that extracellular vesicles play in response to trauma in the central nervous system (CNS). Traumatic brain and spinal cord injury involves a complex yet coordinated multicellular process between CNS intrinsic and extrinsic cells. Increasing evidence suggests that EV-mediated signaling is critical to many mechanisms after neurotrauma, especially local and systemic inflammation (see Dickens et al., 2017). Our lab has previously shown that activated microglia – a key pathological feature in neurotrauma and neurodegenerative disease – release EVs with pro-inflammatory components that are sufficient to activate normal, resting microglia (see Kumar et al., 2017). Sustained microglial activation through EVs may contribute to further secondary neuronal cell death after CNS injury.
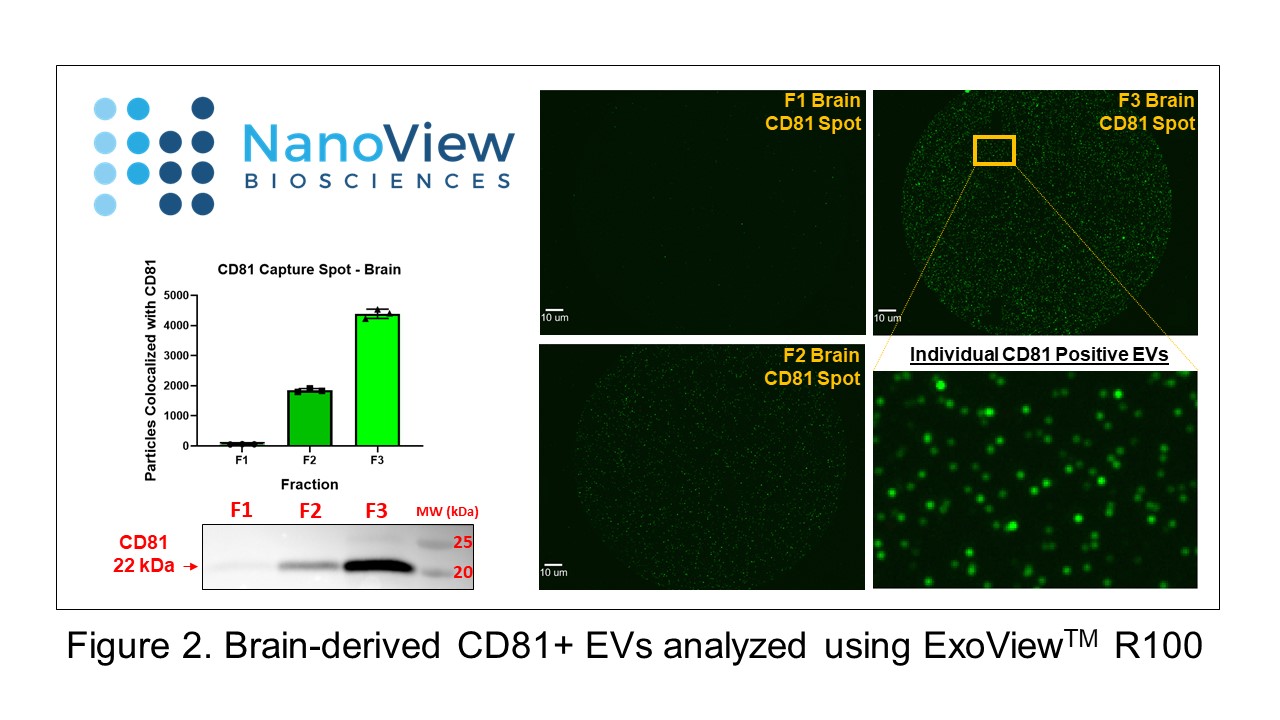
Active research efforts in the lab aim to isolate, characterize, and analyze the functional role of EVs from different cell types in blood, cerebrospinal fluid, and ex vivo tissue in rodent models of traumatic CNS injury. To meet this objective, we use a multi-faceted approach with state-of-the-art technologies including nanoparticle tracking analysis (ViewSizerTM 3000), flow cytometry, and single-particle interferometric reflectance imaging (ExoViewTM R100). Also, in an exciting cross-campus collaboration with College Park, we are working to develop engineered extracellular vesicles as therapeutic agents for the treatment of CNS injury.