Traumatic brain injury (TBI) induces a series of delayed secondary biochemical and cellular changes that contribute to irreversible tissue damage. Delayed secondary biochemical and cellular changes after TBI continue for months to years and are associated with chronic neuroinflammation and progressive neurodegeneration. Increasing evidence suggests that both pathophysiological changes and neurological recovery after injuries to the central nervous system can be modulated by physical exercise. The mechanisms involved may include up-regulation of neurotrophic factors leading to enhanced neuronal plasticity as well as anti-apoptotic and anti-inflammatory effects. Interestingly, an important variable appears to be the timing of initiation of exercise as a function of injury severity which can affect the neurotrophic factor response to injury. Our recent work demonstrates that late exercise initiation beginning at 5 weeks after experimental trauma, but not early initiation of exercise at 1 week, significantly reduces working and retention memory impairments at 3 months, and decreases lesion volume. The improvement in cognitive recovery is associated with attenuation of classical inflammatory pathways, activation of alternative inflammatory responses and enhancement of neurogenesis. In contrast, early initiation of exercise does not alter behavioral recovery or lesion size and increases the neurotoxic pro-inflammatory responses. Our ongoing research underscores the critical importance of timing of exercise initiation after trauma and its relation to neuroinflammation and challenges the widely held view that effective neuroprotection requires early intervention.
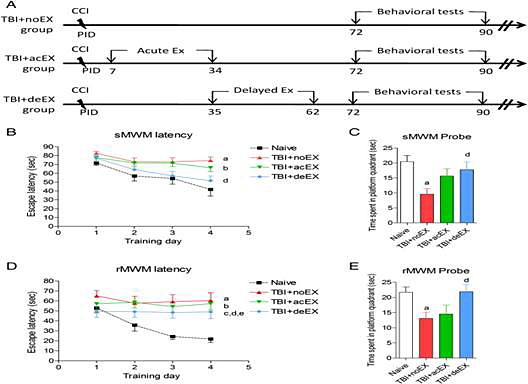
Figure 1: (A.) Experimental protocol. (B.) TBI-induced spatial learning and working memory deficits were assessed using a standard Morris Water Maze (sMWM) test. Injured non-exercised mice (a; p < 0.05 vs. naïve) and acute exercised mice (b; p < 0.05 vs. naïve) showed significant impairment in working memory over time. Injured delayed exercised mice were not significantly different from naïve and showed significant improvements in working memory as compared to non-exercised animal (d; p < 0.05 vs. TBI + noEX). (C.) Reference memory was assessed using the sMWM probe test. There was a significant impairment in reference memory in the non-exercised injured animals (a; p < 0.01 vs. naïve) and a significant improvement in reference memory in injured delayed exercised mice compared to non-exercised animals (d; p < 0.05 vs. TBI + noEX). (D.) Reversal spatial learning was assessed using a Reverse Morris Water Maze (rMWM) test immediately after sMWM. All injured mice showed significantly increased latency to find the sub-merged hidden platform (a-TBI + noEX, b-TBI + acEX, c-TBI + deEX; p < 0.05 vs. naïve). Injured delayed exercise mice showed significant improvements in reversal spatial learning as compared to non-exercised (d; p < 0.05 vs. TBI + noEX) or acute exercise animals (e; p < 0.05 vs. TBI + acEX). (E) Reference memory in rMWM was assessed on the day after the fourth acquisition day. There was a significant impairment in reference memory in the non-exercised injured animals (a; p < 0.01 vs. naïve) and a significant improvement in reference memory in injured delayed exercised mice compared to non-exercised animals (d; p < 0.05 vs. TBI + noEX). Mice spent similar time with the two identical objects during the sample phase in each group.
