October 10, 2023 | Andrew Lentini
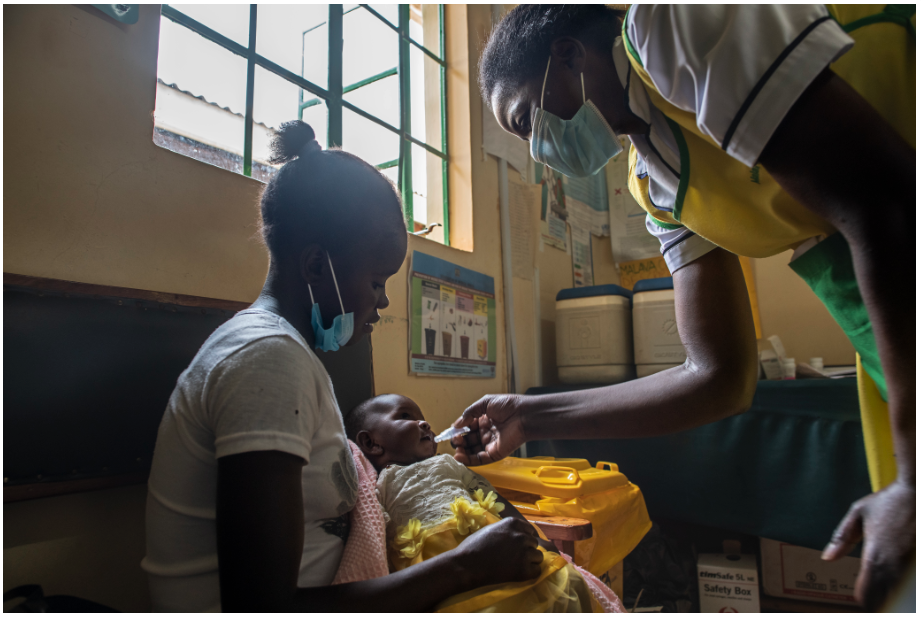
A nurse administers a rotavirus vaccine in Kakamega County, Kenya. Photo: PATH/Anthony Karumba.
Introduction
Diarrheal diseases are the second leading cause of death in children under 5 years after the neonatal period,. Vaccines against rotavirus, historically the most common cause of severe diarrheal illness and death, are being introduced into high-risk settings. Despite reductions in diarrheal disease and associated mortality, nearly 500,000 children continue to die from diarrheal diseases before their 5th birthday, especially those in sub-Saharan Africa. The Vaccine Impact on Diarrhea in Africa (VIDA) Study, funded by a $20 million grant from the Bill & Melinda Gates Foundation to Dr. Karen Kotloff, described the landscape of diarrheal diseases in these high mortality settings. In this report, we discuss the initial findings of VIDA now presented in an 18-article supplement appears in the journal Clinical Infectious Diseases.
Background
CVD investigators were uniquely positioned to conduct VIDA after having conducted a similar study of moderate-to-severe diarrheal (MSD) known as the Global Enteric Multicenter Study (GEMS). The ground-breaking findings from GEMS informed decisions about the interventions most needed to meet goals for reducing diarrhea-related mortality globally. The VIDA Study was conducted using comparable methods to GEMS examine episodes at three GEMS sites in sub-Saharan Africa (Mali, The Gambia and Kenya) that had introduced rotavirus vaccine to provide a contemporary understanding of the burden, causes and outcomes of MSD.
Etiology and Incidence
The supplement articles describe the epidemiology and clinical severity of the major pathogens found to cause diarrhea in these populations, including Shigella, Salmonella, norovirus and other viral agents, Cryptosporidium, Giardia, and diarrheagenic E. coli. Understanding the etiology of diarrheal diseases is paramount for prioritizing preventive strategies. The contributions of breeches in water and sanitation to pathogen-specific infections were explored.
Clinical Impact of Diarrheal Diseases
A pivotal aim of the VIDA Study was to assess the clinical consequences of diarrheal. The study showed that after an episode of MSD, children were 30% more likely to become stunted compared to their matched controls. Linear growth faltering after an episode of MSD was just as likely to occur in VIDA after rotavirus vaccine introduction as in GEMS prior to vaccine introduction. Moreover, nearly 10% of MSD episodes became persistent, particularly those attributed to Cryptosporidium, novovirus, and Shigella. Genetic risk factors for rotavirus infection were also explored.
Management of Diarrhea
We examined adherence to the World Health Organization (WHO) facility-based diarrhea treatment guidelines and found that despite improvements between GEMS and VIDA, most children with dehydration did not receive Oral Rehydration Solutions (ORS) or zinc. Moreover, many children received inappropriate or unnecessary antibiotics.
Child Survival
While there is room for improvement, the supplement also gives cause for celebration. The VIDA Study demonstrated a 65% reduction in diarrheal mortality from GEMS to VIDA at our study sites. The drivers of these declines were multifactorial, including reductions in wasting and increased uptake of rotavirus vaccine, zinc and ORS.
Collaborative Contributions
The VIDA study represented a close and fruitful collaboration between Dr. Kotloff’s team at the University of Maryland School of Medicine Center for Vaccine Development and Global Health, the teams of investigators and staff at each of the sites, and external collaborators at the University of Virginia, the Center for Disease Control and Prevention, and others. Mentorship and transfer of technology to the sites were a high priority as reflected in the high-quality papers coming from the site investigators in this supplement.
Conclusions
The VIDA Study has provided critical insights into the epidemiology and prevention of diarrhea diseases in African sites with high coverage of rotavirus vaccine. These findings are a call to action to increase coverage of interventions that are highly effective in reducing diarrheal morbidity and mortality such as rotavirus vaccine, ORS, and zinc; to reinforce judicious use of antibiotics that are effective and reduce pressure to develop antimicrobial resistance, and to develop new interventions against the key drivers of poor outcomes such as Shigella, Cryptosporidium and norovirus.
For more details about the VIDA Study and related research, you can explore the Volume 76, Issue Supplement_1 of Clinical Infectious Diseases from April 2023.
-
-
The Full Impact of Rotavirus Vaccines in Africa Has Yet to Be Realized
-
A Description of the Statistical Methods for the Vaccine Impact on Diarrhea in Africa (VIDA) Study
-
Drivers of Decline in Diarrhea Mortality Between GEMS and VIDA Studies
-
Shigella in Africa: New Insights From the Vaccine Impact on Diarrhea in Africa (VIDA) Study
-
Contact
Office of Public Affairs
655 West Baltimore Street
Bressler Research Building 14-002
Baltimore, Maryland 21201-1559
Contact Media Relations
(410) 706-5260