April 27, 2020 | Joanne Morrison
Researchers are Testing if Hydroxychloroquine Therapy Can Prevent Infection and Symptoms in Individuals Exposed to COVID-19-Positive Patients
 Researchers at the University of Maryland School of Medicine (UMSOM) have begun testing the effectiveness of hydroxychloroquine as a therapy to prevent infection and symptoms in individuals who have been exposed to COVID-19-positive individuals. The trial is significant because it focuses on preventing COVID-19 and does not involve individuals who are ill with infection but rather healthy individuals who have been exposed.
Researchers at the University of Maryland School of Medicine (UMSOM) have begun testing the effectiveness of hydroxychloroquine as a therapy to prevent infection and symptoms in individuals who have been exposed to COVID-19-positive individuals. The trial is significant because it focuses on preventing COVID-19 and does not involve individuals who are ill with infection but rather healthy individuals who have been exposed.
The research is part of a national study being conducted across the COVID-19 Therapeutics Accelerator, an initiative launched by the Bill & Melinda Gates Foundation (BMGF), Wellcome, and Mastercard, with funding from an array of public and philanthropic donors, to speed up the response to the COVID-19 pandemic by funding the identification, assessment, development and scale up of treatments.
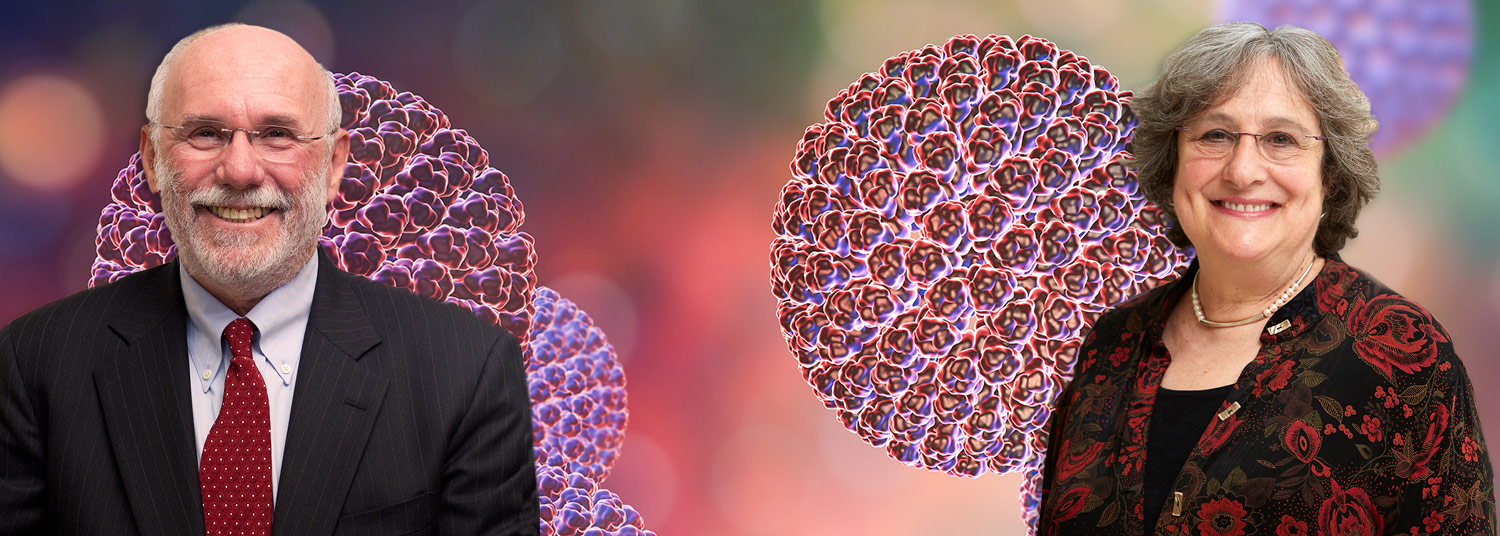
Principal investigators for the COVID-19 Post-Exposure Prophylaxis (PEP) trial – which is being conducted remotely among volunteers throughout the Baltimore-Washington area – are Kathleen Neuzil, MD, MPH, the Myron M. Levine, MD, DTPH, Professor in Vaccinology, Professor of Medicine and Pediatrics, Director of the Center for Vaccine Development and Global Health (CVD), and Miriam Laufer, MD, MPH, Professor of Pediatrics and Associate Director of the CVD’s Malaria Research Program.
 Both Dr. Neuzil and Dr. Laufer are infectious disease specialists, with extensive experience in vaccine and infectious disease research. Dr. Laufer, is a hydroxychloroquine expert, having spent years researching the therapy’s effectiveness in children, pregnant women and people living with HIV, as well as the epidemiology of drug-resistant malaria.
Both Dr. Neuzil and Dr. Laufer are infectious disease specialists, with extensive experience in vaccine and infectious disease research. Dr. Laufer, is a hydroxychloroquine expert, having spent years researching the therapy’s effectiveness in children, pregnant women and people living with HIV, as well as the epidemiology of drug-resistant malaria.
“We know that many COVID-19-positive individuals have mild or no symptoms but are still very contagious. If we can prevent infection or symptoms in individuals who have been exposed to them, we can significantly alter the course of this pandemic,” said Dr. Neuzil.
“Our goal is to reach adult family and household members who live with someone who has recently been diagnosed with COVID-19,” said Dr. Laufer. “To be able to really see how to best protect these exposed individuals, we need to enroll as many people as we can into this study. We want to work with testing sites, public health facilities, medical practices, long-term care facilities and hospitals to identify anyone who might be eligible. Most COVID-19 infection is transmitted within households, so we are working to protect the health of the individuals in the family and limit the broader spread of COVID-19 infection. This could serve as a critical preventative treatment.”
The research is critical as more than three quarters of COVID-19 transmissions occur through close contact within households. The study is being conducted remotely through online video calls and by answering questions via email. Individuals who qualify for the randomized study will take either hydroxychloroquine or a placebo daily for 14 days. Volunteers who participate in the study will be asked to take the medication, complete an online survey to assess their symptoms, and collect a sample by swabbing the inside of their nose every day for 14 days. On Day 28 a final swab will be collected and a survey completed.
 “This research at the University of Maryland School of Medicine is critically important in understanding how we can prevent serious illness from COVID-19. Importantly, a preventative therapy would protect first responders and frontline workers in health facilities and elsewhere. If we can prevent COVID-19 infection, we can help reduce infection and mortality among our most vulnerable individuals,” said Dean E. Albert Reece, MD, PhD, MBA, who is also Executive Vice President for Medical Affairs, UM Baltimore, and the John Z. and Akiko K. Bowers Distinguished Professor, University of Maryland School of Medicine.
“This research at the University of Maryland School of Medicine is critically important in understanding how we can prevent serious illness from COVID-19. Importantly, a preventative therapy would protect first responders and frontline workers in health facilities and elsewhere. If we can prevent COVID-19 infection, we can help reduce infection and mortality among our most vulnerable individuals,” said Dean E. Albert Reece, MD, PhD, MBA, who is also Executive Vice President for Medical Affairs, UM Baltimore, and the John Z. and Akiko K. Bowers Distinguished Professor, University of Maryland School of Medicine.
This research is part of a larger national study led by the University of Washington. This study is a randomized, multi-center study, enrolling nationwide up to 2,000 men and women who meet the eligibility criteria. For more information visit www.covid19pepstudy.org.
 “Our goal is to stop transmission of COVID-19 in the community,” said the multi-site study’s principal investigator Ruanne Barnabas, MBChB, DPhil, Associate Professor of Global Health, University of Washington, “We currently don’t know if hydroxychloroquine works, but through this study we will learn in as short a timeframe as possible whether hydroxychloroquine can prevent transmission in people exposed to the virus.”
“Our goal is to stop transmission of COVID-19 in the community,” said the multi-site study’s principal investigator Ruanne Barnabas, MBChB, DPhil, Associate Professor of Global Health, University of Washington, “We currently don’t know if hydroxychloroquine works, but through this study we will learn in as short a timeframe as possible whether hydroxychloroquine can prevent transmission in people exposed to the virus.”
About the University of Maryland School of Medicine
Now in its third century, the University of Maryland School of Medicine was chartered in 1807 as the first public medical school in the United States. It continues today as one of the fastest growing, top-tier biomedical research enterprises in the world -- with 45 academic departments, centers, institutes, and programs; and a faculty of more than 3,000 physicians, scientists, and allied health professionals, including members of the National Academy of Medicine and the National Academy of Sciences, and a distinguished two-time winner of the Albert E. Lasker Award in Medical Research. With an operating budget of more than $1.2 billion, the School of Medicine works closely in partnership with the University of Maryland Medical Center and Medical System to provide research-intensive, academic and clinically based care for nearly 2 million patients each year. The School of Medicine has more than $540 million in extramural funding, with most of its academic departments highly ranked among all medical schools in the nation in research funding. As one of the seven professional schools that make up the University of Maryland, Baltimore campus, the School of Medicine has a total population of nearly 9,000 faculty and staff, including 2,500 student trainees, residents, and fellows. The combined School of Medicine and Medical System (“University of Maryland Medicine”) has an annual budget of nearly $6 billion and an economic impact more than $15 billion on the state and local community. The School of Medicine faculty, which ranks as the 8th highest among public medical schools in research productivity, is an innovator in translational medicine, with 600 active patents and 24 start-up companies. The School of Medicine works locally, nationally, and globally, with research and treatment facilities in 36 countries around the world. Visit medschool.umaryland.edu.
About the Center for Vaccine Development and Global Health
For over 40 years, researchers in the Center for Vaccine Development and Global Health have worked domestically and internationally to develop, test, and deploy vaccines to aid the world’s underserved populations. CVD is an academic enterprise engaged in the full range of infectious disease intervention from basic laboratory research through vaccine development, pre-clinical and clinical evaluation, large-scale pre-licensure field studies, and post-licensure assessments. CVD has worked to eliminate vaccine-preventable diseases. CVD has created and tested vaccines against cholera, typhoid fever, paratyphoid fever, non-typhoidal Salmonella disease, shigellosis (bacillary dysentery), Escherichia coli diarrhea, nosocomial pathogens, tularemia, influenza, malaria and other infectious diseases. CVD’s research covers the broader goal of improving global health by conducting innovative, leading research in Baltimore and around the world. CVD researchers are developing new and improved ways to diagnose, prevent, treat, control, and eliminate diseases of global impact. Currently, these diseases include typhoid, Shigella, E. coli diarrhea, malaria, and other vaccine-preventable infectious diseases. CVD researchers have been involved in critical vaccine development for emerging pathogens such as Ebola and Zika. In addition, CVD’s work focuses on the ever-growing challenge of antimicrobial resistance.em
Contact
Joanne Morrison
Director of Marketing and Public Relations
University of Maryland School of Medicine
jmorrison@som.umaryland.edu
Office: (410) 706-2884
Mobile: (202) 841-3369