Translational Genomics Laboratory
Mission
The mission of the TranslationalGenomics Laboratory (TGL) is to provide a critical bridge to help accelerate translation from discovery into precision health care, by providing DNA sequencing, genotyping and array-based technologies in both a basic research and clinically-regulated environment.
About the TGL
The Translational Genomics Laboratory (TGL) is the result of 35 years of core facility evolution, starting with peptide and oligonucleotide synthesis, to the current CAP (College of American Pathologists) accredited and CLIA (Clinical Laboratory Improvement Amendments) compliant laboratory, capable of providing support to clinical and translational genetic/genomics studies (CAP# 8017554; CLIA# 21D2027356).
TGL is part of the University of Maryland School of Medicine’s Marlene and Stewart Greenebaum Comprehensive Cancer Center (UMGCC), the Program for Personalized and Genomic Medicine (PPGM), and the Center for Innovative Biomedical Resources (CIBR).
The services offered by the TGL support basic research. The clinical assays offered are similar to those offered to support basic research, except that these assays are validated under CLIA, enabling them to be used in the clinical decision-making process in research protocols and for routine patient care.
Services (Clinical)
- Next Gen Sequencing (NGS) Myeloid Malignancy Targeted Panel
- Confirmation of a Research Finding
- CYP2C19 Genotyping
- Cytogenomic Microarray
- FLT3 ITD and TKD Fragment Size Analysis
- IDH1 R132 and IDH2 R140, R172 Sanger Sequencing
Services (Basic Research)
- Cytogenomic Arrays
- Extraction of Nucleic Acid
- DNA
- RNA
- Gene Expression Arrays
- Global Expression Profiling
- miRNA Expression Profiling
- Transcriptome Analysis
- Genotyping
- Taqman Assays
- Next Generation Sequencing (NGS) Gene Panels
- Sanger DNA Sequencing
Instrumentation
- Applied Biosystems 3730XL (Sanger sequencing)
- Affymetrix GeneChip system 3000 7G (chip-based arrays, e.g. CytoScan HD, DMET)
- Applied Biosystems 9700 thermocycler (PCR amplification)
- Nanodrop single-channel and 8-channel spectrophotometers
- ThermoFisher QuantStudio 5
- Thermofisher Ion Chef System
- Thermofisher Ion S5 Sequencer
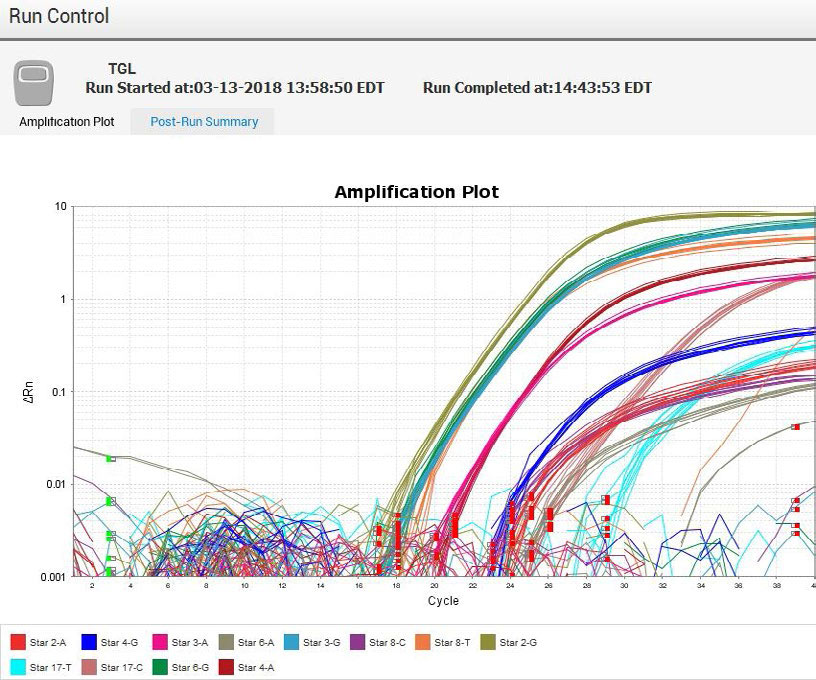
Quant Studio 5 Amplication Plot
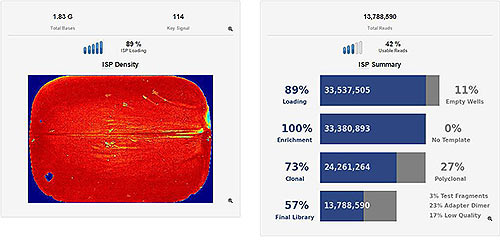
Quant Studio 5 Experiment

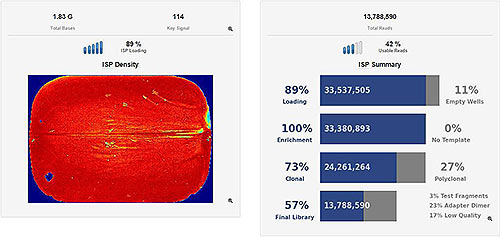
ISP Loading/Run Summary
Forms and Documents
CLIA Certificate of Accreditation 2024 to 2026
QA-506 Clinical Services Test Requisition
QA-514 Clinical Services Test Requisition Extract and Hold Only