CONTACT:

Thomas Blanpied, PhD
Director
Department of Physiology
Room 5-015, Bressler Research Building
tblanpied@som.umaryland.edu
410-706-4769
Joseph Ryan H. Mauban, PhD
Confocal Core Manager
Room 523, Howard Hall
jmauban@umaryland.edu
410-706-6170

Shilpa Dilip Kumar, PhD
Assistant Professor
Room 584, Howard Hall
SDKumar@som.umaryland.edu
Yajie (Kevin) Liang, MB, PhD
Assistant Professor
Department of Diagnostic Radiology and Nuclear Medicine
HSF III, Room L127 Y
yajie.liang@som.umaryland.edu
LOCATION:
Room 610, Health Sciences Facility l
685 West Baltimore Street
Baltimore, MD 21201
HOURS:
Monday through Friday
7:00am - 5:00pm
PHONE:
410-706-6170
Mission
The Confocal Core’s mission is to provide researchers with a wide array of state-of-the-art confocal imaging equipment to enable acquisition of high resolution images (both in vivo and in vitro). The Confocal Core offers training and assistance in the use of multiple confocal microscopes housed in our facility. Optimization of data acquisition and image processing are both part of the training, thus enabling researchers to efficiently design studies, acquire image data and extract relevant data features. The confocal facility is available to all UMB researchers and extramural users on a fee-for-service basis.
Services
The facility provides individual instruction on an array of confocal microscopes. The needs of the researcher are considered in choosing which microscope will best suit the experimental design. In general, imaging of fixed samples, cultured cells, organ slices and small animals can be accommodated. Imaging techniques including FRET, FRAP, photoactivation and uncaging are readily implemented. All microscopes are well suited to multicolor imaging of common fluorophores, and two microscopes are also equipped with multiphoton excitation suitable for in vivo imaging. An image analysis workstation equipped with software packages is available to users.
The Core also has a culture room with an incubator, culture hood, and a widefield fluorescence microscope for use in preparation of cultured and live samples. Preparation of live animals for imaging experiments can also be done in this newly renovated space.
Instrumentation
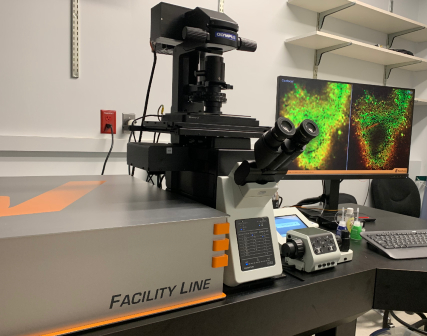 Abberior Facility Line STED Microscope
Abberior Facility Line STED Microscope
- Super-resolution STED microscope capable of lateral resolution ~40 nm and axial resolution ~80 nm.
- Suitable for super-resolution imaging in cultured cells, tissue slices, and other preparations.
- Fluorescence Lifetime Imaging (FLIM) for FRET and other new measurements.
- Core hardware for image acquisition
- Laser lines: 405, 485, 561 and 640 nm for imaging and a pulsed STED laser at 775nm.
- Detection via 3 photon-counting APDs and 1 Matrix detector for effective removal of background signals.
- Inverted Olympus IX83 with 10x (dry), 20x (dry), 40x (dry), 60x (silicone oil) and 60x (oil) objectives for a broad range of applications, motorized stage, and Z drift compensation.
- Capable of imaging fixed and live samples.
- Okolab stage-top incubator for temperature and CO2 control for live-cell imaging.
- A range of sample holders is available.
- STED-capable live-cell stains are available for a variety of intracellular targets.
- Unique accessories to increase resolution, decrease photobleaching, and:
- Adaptive optics to correct for spherical aberrations and compensate for sample-induced aberrations.
- Adaptive Illumination that facilitates high resolution and low-light gentle imaging of live-cells.
- Novel approaches called DyMIN and RESCue that reduce the total light power delivered to the sample during image scanning.
 Zeiss LS7 Lightsheet microscope
Zeiss LS7 Lightsheet microscope
- Lightsheet microscope for visualization of large 2d and 3d samples
- Six laser lines: 405, 445, 488, 514, 561, 638
- Illumination optics; 5x/0.1 foc, 10x/0.2 foc
- Detection objectives for clearing and water-based samples (2.5x, 5x, 10x, 20x)
- Water chamber with temperature control
- Mesoscale chamber (Translucence Biosystems)
- 2 PCO Edge sCMOS cameras
- Zeiss, Arivis, Imaris Bitplane analysis software
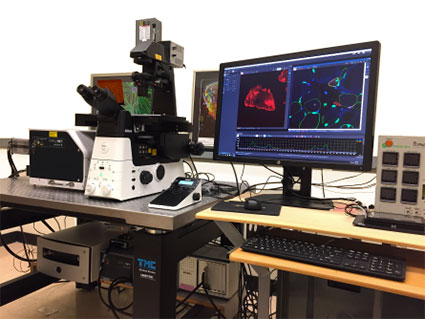 Nikon W1 spinning disk
Nikon W1 spinning disk
- Spinning disk confocal on Nikon Ti2 inverted microscope
- Excellent for high speed and live-cell 3D imaging of cells and sections
- Equipped with an incubation chamber for live samples
- Motorized stage and software for automated tiling, stitching, and reconstruction
- 7 laser lines for multicolor imaging
- 2 Hamamatsu sCMOS camera for high resolution imaging
- DMD equipped
- TIRF optics
Nikon A1 Laser Confocal
- Point-scanning laser confocal on inverted Ti2 microscope
- Excellent for immunocytochemistry with advanced tiling and stitching capabilities
- 4 laser and 4 high-sensitivity detectors for multicolor imaging
- Conveniently located in HSF3
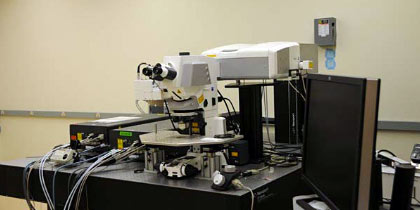 Zeiss 710 NLO & Zeiss 7MP
Zeiss 710 NLO & Zeiss 7MP
- Two separate, upright microscope systems equipped with multiphoton excitation for imaging live cells, slices and whole animals
- 710 NLO equipped with a confocal LSM and spectral detection
- 7MP equipped with two lasers for imaging a very broad range of fluorophores.
- Each equipped with 4 detectors for high-sensitivity multicolor imaging
- 7MP is ideal configuration for in vivo imaging
 Zeiss 5Live & Zeiss 510
Zeiss 5Live & Zeiss 510
- Point-scanning and slit-scanning confocal microscope
- Fast acquisition frame rates for studying dynamic cellular processes at physiological temperatures
- Dual scan heads (5Live) allow simultaneous imaging and optical manipulation
- Excitation (488, 543, 560, 633, Ti:Sapphire laser)
 Olympus LCV Incubated Microscope
Olympus LCV Incubated Microscope
- Widefield inverted microscope allowing continuous imaging of cells for hours or days
- Fluorescence and DIC imaging on multiple positions
- Cell migration, cell division, wounding and repair processes, phagocytosis
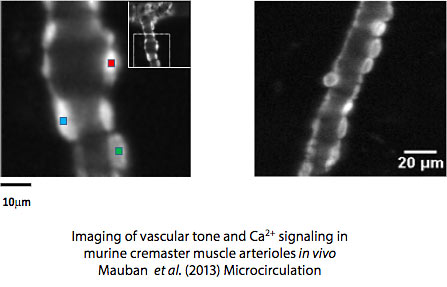 Imaris Bitplane
Imaris Bitplane
Bitplane is an advanced image analysis software for processing images. 3D renditions, display and quantification are readily executed.
Some imaging suites are specialized for certain applications, e.g. neurofilament tracing. Other common processing routines are available. The confocal core operates a floating license server, which allows easy operation of the software from the investigator’s own computers.