Clinical and Translational Research Training Track

Welcome to our newest cohort of CARTI scholars who started with orientation on January 7th!
- Meaghan E. Broderick, MD, Visiting Instructor, Surgery
- Shishir Dahal, MD, PhD, Postdoctoral Fellow, Neurology
- Sheng Feng, PhD, Assistant Professor, Pathology
- Simon K. Ho, PT, DPT, Assistant Professor, Physical Therapy & Rehabilitation Science
- Priyam Jani, PhD, Clinical Assistant Professor, Comprehensive Dentistry, UM School of Dentistry
- Marcel B. Lanza, PhD, Assistant Professor, Physical Therapy & Rehabilitation Science
- Kerri E. Lopez, MD, Assistant Professor, Surgery
- Arissa M. Torrie, MD, MHS, Assistant Professor, Anesthesiology
- Chenchen Zhang, MD, PhD, Assistant Professor, Medicine
Course Timeline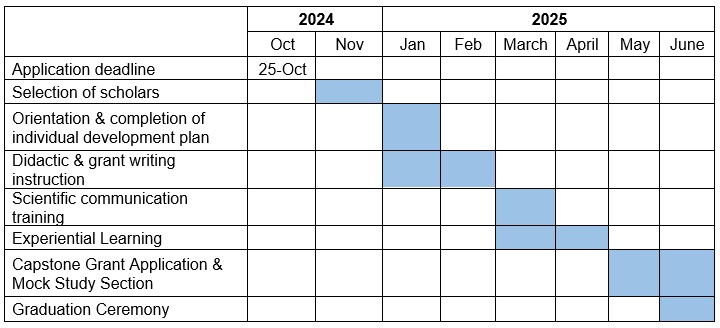
Spring 2025 schedule
|
Week |
Topic |
|
1 |
Welcome & Orientation |
|
1 |
Grant Writing – The Basics |
|
1 |
Developing a clinical research question and hypothesis |
|
1 |
Specific Aims |
|
1 |
Critically reviewing the scientific literature/Rigor of prior research |
|
2 |
Writing & publishing a research manuscript |
|
2 |
Significance & Innovation |
|
2 |
Specific Aims draft |
|
2 |
Specific Aims draft |
|
3 |
Approach/Methods |
|
3 |
Design and Implementation of a clinical research study |
|
3 |
Introduction to study design – quantitative and qualitative research |
|
3 |
Study design: cross sectional studies |
|
3 |
Study design: case-control and cohort studies |
|
4 |
Study design: clinical trials |
|
4 |
Choosing study subjects, sampling, and recruitment strategies |
|
4 |
Significance & Innovation draft |
|
4 |
Significance & Innovation draft |
|
5 |
Identifying study measurements and data collection methods |
|
5 |
Health survey research methods |
|
5 |
Biostatistics basics, interacting with a biostatistician |
|
5 |
Principles of sample size estimation |
|
5 |
Data management |
|
6 |
Giving an effective research presentation/executive presence |
|
6 |
IRB Approval |
|
6 |
Full project presentation |
|
6 |
Full project presentation |
|
6 |
Full project presentation |
|
7 |
Identifying sources of funding |
|
7 |
Project Summary & Public Health Narrative |
|
7 |
NIH biosketch, Letters of Support |
|
7 |
Developing a budget, budget justification, and administrative components of a grant application |
|
7 |
Responding to reviewer critiques |
|
8 |
NIH Biosketch feedback (optional) |
