News

September 6, 2024
Dr. Lin Zou awarded a NIH grant for trauma research
Dr. Lin Zou, Associate Professor of Anesthesiology, has received a research grant from the National Institutes of Health (NIH) for trauma research. Trauma claims for 8% of deaths and 16% of disabilities worldwide. Despite significant improvement in clinical care, overall trauma mortality remains high largely due to trauma-associated organ injury and secondary infection. The 5-year NIH R35 award entitled “Extracellular nucleic acids in trauma innate immunity” will support Dr. Zou’s team to investigate how extracellular nucleic acid such as RNA and DNA, released by injured cells, interacts with the body’s innate immune system and plays a role in inflammation, vascular injury, and multi-organ dysfunction after trauma. By dissecting the molecular mechanism of the trauma immunity, they are hoping to identify novel therapeutic targets to mitigate the secondary organ damage after severe injury. Click the link for additional information.

August 29, 2024
Dr. Brittney Williams named Director of Clinical Research for Anesthesiology
Dr. Brittney Williams has been appointed the Director of Clinical Research, a leadership position newly created to enhance the ongoing clinical research in the Department of Anesthesiology.
Dr. Williams is a board-certified cardiothoracic Anesthesiologist, Associate Professor, and an outstanding physician-scientist. After completing her anesthesiology residency and cardiothoracic fellowship at the UMB in 2016, she devoted the next few years in clinical and laboratory research on coagulation and coagulopathy. Her clinical research has been mainly focused on 1) identification of global clotting abnormalities using viscoelastic (ROTEM) testing during cardiopulmonary bypass, 2) the influence of abnormal coagulant and immune responses in post-bypass prothrombotic states, and 3) evaluation of clinical interventions to limit disturbances in blood coagulation. These clinical studies, published on J Cardiothorac Vasc Anesth., Anesthesiology, Brit J Anesth., and Anesth&Analg., enhance our understanding of coagulopathy related to cardiac surgery and help improve the perioperative care.
Dr. Williams has also developed a competitive basic/translational research program to understand the molecular basis of sepsis-induced coagulopathy (SIC). She and her team developed and fully characterized one of the first murine model of SIC. Her work provided the first evidence that TLR2 and TLR7 are key players in bacterial SIC. Her recent work has been focused on the role of extracellular vesicles in coagulopathy during cardiac bypass surgery and in septic patients, and novel biomarker identification in human sepsis and trauma.
Throughout the past eight years, Dr. Williams has demonstrated a strong commitment to original research and scholarship pursuit. Because of her dedication and excellence, she was awarded the ASA/FAER-mentored research training grant in 2019 and NIH-Clinician Scientist Development Award (K08) in 2020. She also serves as a co-principal investigator on a DoD-funded project to study trauma-induced coagulopathy.
As the Director, Dr. Williams will be tasked to organize the current clinical research program, improve the efficiency and transparency of our clinical research infrastructure, support various clinical research projects, and help identify and train the next generation of physician-scientists in the Department of Anesthesiology.

March 18, 2024
Dr. Brittney Williams and colleagues publish a Featured Article on the April 2024 issue of Anesthesia & Analgesia
.jpeg) Brittney Williams, MD - Sepsis is a serious clinical condition caused by a dysregulated host response to infection and characterized by marked systemic inflammation, organ damage, failing homeostasis, coagulopathy, and ultimately multiple organ failure. In the U.S., sepsis is the 10th leading cause of death and #1 cause of in-hospital mortality. Approximately half of septic patients in ICU have clotting dysfunction, a condition termed sepsis-induced coagulopathy (SIC). The pathogenesis and clinical management of SIC are complex and challenging.
Brittney Williams, MD - Sepsis is a serious clinical condition caused by a dysregulated host response to infection and characterized by marked systemic inflammation, organ damage, failing homeostasis, coagulopathy, and ultimately multiple organ failure. In the U.S., sepsis is the 10th leading cause of death and #1 cause of in-hospital mortality. Approximately half of septic patients in ICU have clotting dysfunction, a condition termed sepsis-induced coagulopathy (SIC). The pathogenesis and clinical management of SIC are complex and challenging.
In a Featured Article published on the April issue of A&A, “Sepsis-Induced Coagulopathy: A Comprehensive Narrative Review of Pathophysiology, Clinical Presentation, Diagnosis, and Management Strategies”, Dr. Brittney Williams and her colleagues take an in-depth look through both preclinical and clinical investigations and critically examine the role of innate immune signaling in the development of coagulopathy, the impact on its clinical presentation, and the major clinical trials of various treatment strategies in SIC. The article provides a rare insight into the basic mechanism and clinical management of the complicated disease. In addition to being the A&A cover story, there is an editor-organized podcast – Article of the Month – focusing on the undertaking of this project and Q/A on sepsis-induced coagulopathy.

August 13, 2022
Danielle Hill, MHA
Congratulations to Danielle Hill on her Master's degree in Health Administration at the University of Maryland, Global Campus.


August 6, 2022
A new study sheds light on the role of host innate immunity in platelet activation during bacterial sepsis.
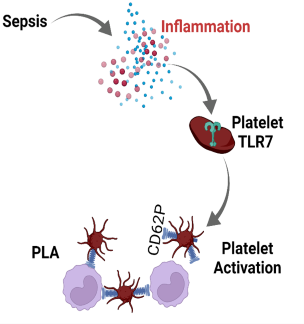 Brittney Williams, MD - Sepsis is a clinical syndrome caused by a dysregulated host response secondary to infection and characterized by marked systemic inflammation and coagulation activation, two processes inextricably linked. Sepsis-induced thrombocytopenia is a common complication in sepsis and is associated with poor prognosis and higher mortality. Dr. Brittney Williams and colleagues from the School of Medicine Department of Anesthesiology Translational Research Program recently investigated the role of innate immune Toll-like-receptor 7 (TLR7), sensor for single-stranded RNA, in platelet activation and platelet leukocyte aggregate (PLA) formation in a murine model of bacterial sepsis. They found that direct stimulation of platelets with known TLR7 agonist triggered platelet release of p-selectin cell adhesive protein that facilitates platelet-leukocyte binding. Loss-of-function study demonstrated that systemic TLR7 deficiency resulted in significantly reduced platelet-leukocyte aggregates and better preservation of platelet counts in sepsis. Notably, platelets were activated upon exposure to septic plasma in part via platelet-TLR7 signaling. These data highlight that circulating plasma mediator(s) in septic mice activate platelets in part via TLR7 and offer new insight into immune-mediated platelet activation in sepsis (see figure). These findings are recently published in Platelets.
Brittney Williams, MD - Sepsis is a clinical syndrome caused by a dysregulated host response secondary to infection and characterized by marked systemic inflammation and coagulation activation, two processes inextricably linked. Sepsis-induced thrombocytopenia is a common complication in sepsis and is associated with poor prognosis and higher mortality. Dr. Brittney Williams and colleagues from the School of Medicine Department of Anesthesiology Translational Research Program recently investigated the role of innate immune Toll-like-receptor 7 (TLR7), sensor for single-stranded RNA, in platelet activation and platelet leukocyte aggregate (PLA) formation in a murine model of bacterial sepsis. They found that direct stimulation of platelets with known TLR7 agonist triggered platelet release of p-selectin cell adhesive protein that facilitates platelet-leukocyte binding. Loss-of-function study demonstrated that systemic TLR7 deficiency resulted in significantly reduced platelet-leukocyte aggregates and better preservation of platelet counts in sepsis. Notably, platelets were activated upon exposure to septic plasma in part via platelet-TLR7 signaling. These data highlight that circulating plasma mediator(s) in septic mice activate platelets in part via TLR7 and offer new insight into immune-mediated platelet activation in sepsis (see figure). These findings are recently published in Platelets.

August 02, 2022
Brittney Williams, MD
Congratulations to Brittney Williams, MD on her promotion to Associate Professor of Anesthesiology at the University of Maryland School of Medicine.


August 1, 2022
A new study reveals the role of extracellular RNA sensing in acute respiratory distress syndrome in sepsis
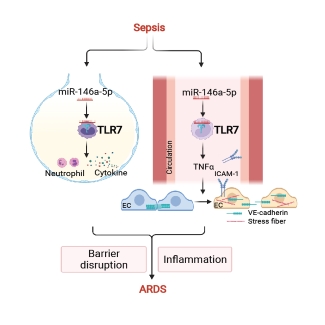 Sepsis-induced acute respiratory distress syndrome (ARDS) causes profound morbidity and mortality worldwide. Pulmonary inflammation and endothelial barrier disruption are pathological hallmarks. A team of scientists led by Dr. Lin Zou from the School of Medicine and Pharmacy of the University of Maryland investigated the role of nucleic acid sensing in these pathological changes. They discovered that intratracheal delivery of microRNA (miR)-146-5p triggered lung inflammation and disrupted alveolar barrier function via Toll-like receptor 7 (TLR7), a single-stranded RNA sensor. Systemic deficiency of TLR7 markedly reduced sepsis-associated lung injury manifested as decreased bronchoalveolar lavage and lung tissue cytokines and preserved pulmonary barrier function. Surprisingly, endothelial injury mediated by the miR-146a-5p-TLR7 pathway appeared attributed to an indirect mechanism likely mediated by miR-146a-5p-TLR7 sensing in immune cells through gap junction proteins VE-cadherin. Finally, cytokine array, pathway enrichment analysis, and neutralizing antibody experiments identified TNFa as the key downstream effector of miR-146a-5p-TLR7 signaling responsible for endothelial injury (See Figure). These findings are recently published in Am J Respir Cell Mol Biol.
Sepsis-induced acute respiratory distress syndrome (ARDS) causes profound morbidity and mortality worldwide. Pulmonary inflammation and endothelial barrier disruption are pathological hallmarks. A team of scientists led by Dr. Lin Zou from the School of Medicine and Pharmacy of the University of Maryland investigated the role of nucleic acid sensing in these pathological changes. They discovered that intratracheal delivery of microRNA (miR)-146-5p triggered lung inflammation and disrupted alveolar barrier function via Toll-like receptor 7 (TLR7), a single-stranded RNA sensor. Systemic deficiency of TLR7 markedly reduced sepsis-associated lung injury manifested as decreased bronchoalveolar lavage and lung tissue cytokines and preserved pulmonary barrier function. Surprisingly, endothelial injury mediated by the miR-146a-5p-TLR7 pathway appeared attributed to an indirect mechanism likely mediated by miR-146a-5p-TLR7 sensing in immune cells through gap junction proteins VE-cadherin. Finally, cytokine array, pathway enrichment analysis, and neutralizing antibody experiments identified TNFa as the key downstream effector of miR-146a-5p-TLR7 signaling responsible for endothelial injury (See Figure). These findings are recently published in Am J Respir Cell Mol Biol.

November 19, 2021
microRNA-TLR7 Signaling Mediates Brain Inflammation during Bacterial Sepsis
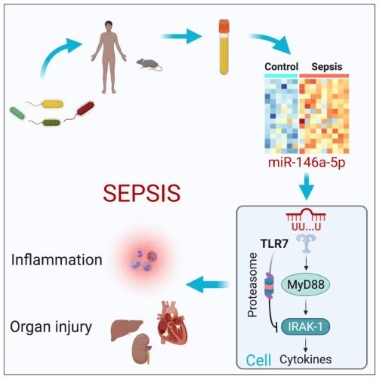
Sepsis is a serious clinical condition with organ dysfunction mediated by a dysregulated host response to infection. Up to 70% of septic patients develop cerebral dysfunction, a condition termed sepsis-associated encephalopathy (SAE). Septic patients with SAE have a higher mortality rate than patients with normal mental status. Among sepsis survivors, many developed long-term cognitive impairment that adversely impacts the quality of their lives. The molecular mechanism underlying SAE is not well understood. A multidisciplinary research team led by Lin Zou, Junfang Wu, Wei Chao at the Center for Shock, Trauma and Anesthesiology Research reported an important role of microRNA®TLR7 sensing in brain innate immune response in murine sepsis. Mice with SAE exhibited brain-blood barrier (BBB) disruption, profound neuroinflammation, and motor coordination, and neurological impairment. Sepsis significantly increased plasma miRNA levels, such as miR-146a-5p. Exogenous miR-146a-5p induces innate immune responses in both cultured microglia/astrocytes and the intact brain via a TLR7-dependent manner. Moreover, miR-146a-/- mice showed reduced accumulation of monocytes and neutrophils in the brain compared to WT after sepsis. Finally, TLR7-/- mice had preserved BBB integrity, reduced microglial expansion, and leukocyte accumulation in the brain, but did not improve neurobehavioral recovery following sepsis. These data establish an important role of extracellular miRNA and TLR7 sensing in sepsis-induced brain inflammation. The study is published online in Brain, Behavior, and Immunity.

November 12, 2021
A new study reveals a critical role of extracellular microRNA in innate immunity and sepsis

September 9, 2021
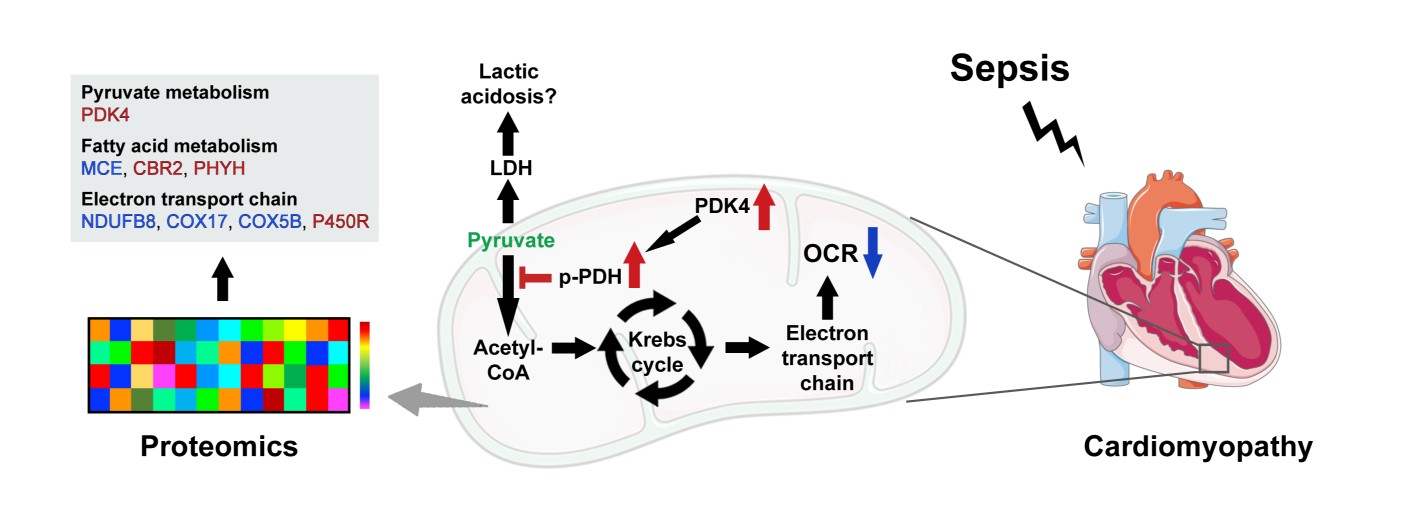
Dissecting mitochondrial metabolic dysfunction linked to sepsis-induced cardiomyopathy

Figure 1:

Lin Zou, MD, PhD
July 01, 2021 - Congratulations to Lin Zou MD, PhD on her promotion to Associate Professor with the University of Maryland, School of Medicine; Department of Anesthesiology.

Wei Chao, MD, PhD
May 1, 2021- Wei Chao, MD, PhD, FAHA, was awarded an R35 grant, $1,931,250.00, from the National Institute of General Medical Sciences (NIH/NIGMS) to study “Extracellular miRNAs, innate immunity, and critical illness”. According to NIH, the goal of the R35 award, also known as Maximizing Investigators' Research Award, is to provide investigators with greater stability and flexibility, thereby enhancing scientific productivity and the chances for important breakthroughs. Dr. Chao's team will investigate the role of innate immunity in sepsis and ischemic myocardial infarction (MI), two critical illnesses with very high mortality. While their etiologies are distinctively different, they share similar features of innate immune activation, marked tissue inflammation, and acute cardiac dysfunction. The specific goal of the R35 project is to delineate the mechanistic role of cell-free miRNAs and extracellular vehicles in innate immune activation and in the pathogenesis of sepsis and myocardial ischemic injury. Click here for more information on this grant.

Wei Chao, MD, PhD
March 22, 2021- Congratulations to both Samuel M. Galvagno, DO, PhD, and Wei Chao, MD, PhD, FAHA as the inaugural awardees of the 2021 Colin Mackenzie and Cristina Imle Mentoring Prize. Professor Emeritus Colin F. Mackenzie, MB, ChB, MD, and his wife, Cristina Imle, PT, made a generous donation to the Department of Anesthesiology to support an Annual Mentoring Prize. The Mentoring Prize recognizes a faculty member(s) who has consistently demonstrated quality mentorship and has impacted professional development and career advancement in one or more of the following areas: clinical medicine, research, local and regional service, and teaching.

Brittney Williams, MD
August 25, 2020 - Brittney Williams, MD awarded the Mentored Clinical Scientist Research Career Development Award. Her project entitled "Role of miRNA-TLR7 Signaling in Platelet Activation and Dysfunction in Sepsis" was approved for funding by the National Heart, Lung, and Blood Institute (NHLBI). Dr. Williams stated she was very pleased with the new NIH support and that her work in the next 3-5 years will focus on delineating platelet signaling pathways involved in immune-mediated thrombotic complications in sepsis.

Kerri Lopez, MD
July 28, 2020- Kerri Lopez, MD awarded the SHOCK Society Diversity Enhancement Award for her abstract entitled "Impact of Hypobaric Exposure on Inflammation and Organ Injury in a Non-Hemorrhagic Mouse Model of Polytrauma". Dr. Lopez is a PGY-3 surgical resident and has just completed her two-year research training in the Anesthesiology Translational Research Program working on a research project funded by the US Air Force.

Kerri Lopez, MD
2020- Kerri Lopez, MD awarded third place in the University of Maryland Department of Surgery Annual Charles Getz Research Retreat for her oral presentation titled “Transport after Traumatic Injury – the Effects of Hypobaria.”

Brittney Williams, MD
2019- Brittney Williams, MD awarded the Mentored Research Training Grant (MRTG) by the Foundation for Anesthesia Education and Research (FAER) for her research project titled "Role of TLR7 in Platelet Activation and Dysfunction in Sepsis". The award, $250,000 over two years, helps physician-scientists develop the skills, preliminary data for subsequent grant applications, and research publications needed to become independent investigators. In 2019, FAER received 58 high-quality applications for research training grants. Dr. Williams was selected as one of the 5 recipients of this prestigious award. Click Here for more information on this award.

Briana Shimada, PhD
2019- Briana Shimada, PhD awarded first place in the Univerisity of Maryland 2019 Shock, Trauma and Anesthesiology Research (STAR) 10-year Celebration for her basic science abstract titled "MIR-146A-5P Mimic Induces Cardiac Inflammation and Dysfunction Via Toll-Like Receptor 7”

Wei Chao, MD, PhD and Lin Zou, MD, PhD
2019- Wei Chao, MD, PhD, Junfang Wu, PhD, and Lin Zou, MD, PhD R01 funded grant titled "Targeting Brain Inflammation and Neurocognitive Dysfunction in Sepsis" from the National Institute of Neurological Disorders and Stroke. Click Here for more information.

Andrew Suen, MD
2019- Andrew Suen, MD awarded first place in the Univerisity of Maryland Department of Anesthesiology 2019 Research Retreat for his basic science poster titled “Circulating Plasma MiRNAs Trigger Innate Immune Activation in a Mouse Model of Polytrauma”

Brittney Williams, MD
2019- Brittney Williams, MD awarded first place in the Univerisity of Maryland Department of Anesthesiology 2019 Research Retreat for her basic science presentation titled "Molecular Link Between Inflammation and Coagulation Dysfunction in Polymicrobial Sepsis"

Wei Chao, MD, PhD
2018- Wei Chao, MD, PhD, named the recipient of the prestigious 2018 Frontiers in Anesthesia Research Award, given by the International Anesthesia Research Society (IARS). This $750,000 award is only given by the IARS once every three years. Dr. Chao will use this funding to support his research into the molecular pathogenesis of sepsis.

Andrew Suen, MD
2018- Andrew Suen, MD for being awarded the Association of University Anesthesiologists (AUA) Resident Travel Award for his oral presentation titled "Extracellular miRNAs and Innate Immune Activation Following Polytraumatic Injury" during the 2018 IARS, AUA, and SOOCA Annual Meetings.

Brittney Williams, MD
2018- Brittney Williams, MD awarded the International Anesthesia Research Society’s (IARS) Kosaka Best of Meeting Abstract Award Finalists for her abstract titled “Role of Toll-like Receptor Signaling in Sepsis-induced Coagulopathy in Mice”

Lin Zou, MD, PhD
2017- Lin Zou, MD, PhD received R35 funded grant titled "Function and Mechanisms of extracellular microRNAs in sepsis-induced lung injury" from the National Institute of General Medical Sciences. Click Here for more information.

Lin Zou, MD, PhD
2017- Lin Zou, MD, PhD awarded the prestigious Faculty Research Award from the Shock Society. The award is given in support of a faculty member at his/her early career stage to develop research in the areas related to trauma, shock, and sepsis, and who has the intent to become an outstanding independent investigator.
Contact
Office: 410-328-9461 or 410-706-2566
Lab: 410-706-2548 or 410-706-2549
Email: wchao@som.umaryland.edu
