
Bogdan Stoica, MD
Email: Bstoica@som.umaryland.edu
(410) 706 5186
Overview:
My research mainly focuses on understanding the molecular mechanisms of neuronal cell death and neuroinflammation after central nervous system trauma. My studies are based on the hypothesis that brain trauma initiates multiple maladaptive mechanisms (secondary injury) that lead to improper activation of neuronal cell death pathways and/or prevent efficient activation of neuronal repair mechanisms. Thus, neurons that receive a survivable injury are unnecessarily removed and/or fail to undergo effective repair/regeneration. An essential driver of these changes is injury-induced dysregulation of microglia responses that shift the microglia post-injury reactive states toward specific persistent pro-inflammatory phenotypes, resulting in secondary neurotoxicity.
Areas of special interest include: 1) the modulation of secondary injury mechanisms by microRNAs, a group of regulatory non-coding small RNA molecules following experimental traumatic brain injury (TBI); our recent data suggest that injury-induced changes in specific microRNAs are key to the activation of neuronal cell death pathways and ultimately to neuronal cell loss after TBI; and 2) the transcriptomic changes and their epigenetic underpinning that drive the molecular and cellular secondary injury processes.
By identifying the injury-induced molecular dysfunctions, we can design optimal therapeutic approaches that will shift microglia activation toward neurorestorative phenotypes to increase neuronal survival and recovery after brain trauma, thus improving neurological deficits.
Lab Overview
Dr. Stocia's research continues to focus on the mechanisms of secondary injury following experimental TBI, including neuronal cell death and neuroinflammation and the identification of effective therapeutic interventions.
Projects
Inhibition of chronic neuroinflammation reduces neurological deficits after TBI
Traumatic brain injury (TBI) induces a series of delayed secondary biochemical and cellular changes that contribute to irreversible tissue damage. Delayed secondary biochemical and cellular changes after TBI continue for months to years and are associated with chronic neuroinflammation and progressive neurodegeneration. Increasing evidence suggests that both pathophysiological changes and neurological recovery after injuries to the central nervous system can be modulated by physical exercise. The mechanisms involved may include up-regulation of neurotrophic factors leading to enhanced neuronal plasticity as well as anti-apoptotic and anti-inflammatory effects. Interestingly, an important variable appears to be the timing of initiation of exercise as a function of injury severity which can affect the neurotrophic factor response to injury. Our recent work demonstrates that late exercise initiation beginning at 5 weeks after experimental trauma, but not early initiation of exercise at 1 week, significantly reduces working and retention memory impairments at 3 months, and decreases lesion volume. The improvement in cognitive recovery is associated with attenuation of classical inflammatory pathways, activation of alternative inflammatory responses and enhancement of neurogenesis. In contrast, early initiation of exercise does not alter behavioral recovery or lesion size and increases the neurotoxic pro-inflammatory responses. Our ongoing research underscores the critical importance of timing of exercise initiation after trauma and its relation to neuroinflammation and challenges the widely held view that effective neuroprotection requires early intervention.
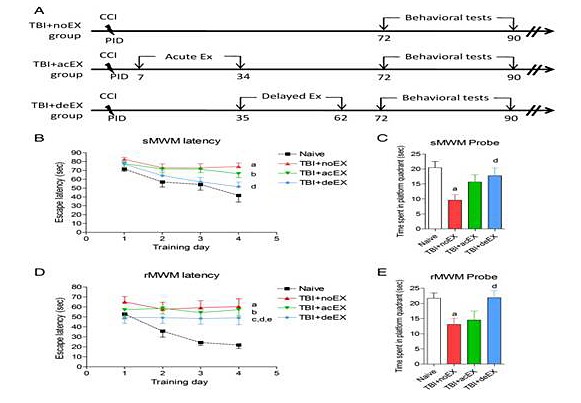
Figure 1: (A.) Experimental protocol. (B.) TBI-induced spatial learning and working memory deficits were assessed using a standard Morris Water Maze (sMWM) test. Injured non-exercised mice (a; p < 0.05 vs. naïve) and acute exercised mice (b; p < 0.05 vs. naïve) showed significant impairment in working memory over time. Injured delayed exercised mice were not significantly different from naïve and showed significant improvements in working memory as compared to non-exercised animal (d; p < 0.05 vs. TBI + noEX). (C.) Reference memory was assessed using the sMWM probe test. There was a significant impairment in reference memory in the non-exercised injured animals (a; p < 0.01 vs. naïve) and a significant improvement in reference memory in injured delayed exercised mice compared to non-exercised animals (d; p < 0.05 vs. TBI + noEX). (D.) Reversal spatial learning was assessed using a Reverse Morris Water Maze (rMWM) test immediately after sMWM. All injured mice showed significantly increased latency to find the sub-merged hidden platform (a-TBI + noEX, b-TBI + acEX, c-TBI + deEX; p < 0.05 vs. naïve). Injured delayed exercise mice showed significant improvements in reversal spatial learning as compared to non-exercised (d; p < 0.05 vs. TBI + noEX) or acute exercise animals (e; p < 0.05 vs. TBI + acEX). (E) Reference memory in rMWM was assessed on the day after the fourth acquisition day. There was a significant impairment in reference memory in the non-exercised injured animals (a; p < 0.01 vs. naïve) and a significant improvement in reference memory in injured delayed exercised mice compared to non-exercised animals (d; p < 0.05 vs. TBI + noEX). Mice spent similar time with the two identical objects during the sample phase in each group.
Publications
Barrett JP, Aubrecht TG, Smith A, Vaida M, Henry RJ, Doran SJ, Faden AI, Stoica BA. Molecular Pathway Changes Associated with Different Post-Conditioning Exercise Interventions After Experimental TBI. J Neurotrauma. 2024 Aug 21. doi: 10.1089/neu.2024.0120; PMID: 39078326.
Henry RJ, Barrett JP, Vaida M, Khan NZ, Makarevich O, Ritzel RM, Faden AI, Stoica BA. Interaction of high fat diet and brain trauma alters adipose tissue macrophages and brain microglia associated with exacerbated cognitive dysfunction. J Neuroinflammation. 2024 Apr 29;21(1):113.; PMID: 38685031.
Sabirzhanov B, Makarevich O, Barrett J, Jackson IL, Faden AI, Stoica BA. Down-Regulation of miR-23a-3p Mediates Irradiation-Induced Neuronal Apoptosis. Int J Mol Sci. 21(10). 2020 May 24;21(10):3695. PMID:32456284.
Sabirzhanov B, Makarevich O, Barrett JP, Jackson IL, Glaser EP, Faden AI, Stoica BA. Irradiation-Induced Upregulation of miR-711 Inhibits DNA Repair and Promotes Neurodegeneration Pathways. Int J Mol Sci. 2020 Jul 23;21(15):5239. PMID: 32718090.
Makarevich O, Sabirzhanov B, Aubrecht TG, Glaser EP, Polster BM, Henry RJ, Faden AI, Stoica BA. Mithramycin selectively attenuates DNA-damage-induced neuronal cell death. Cell Death Dis. 2020 Jul27;11(7):587. PMID: 32719328.
Interests
Areas of special interest include: 1) the modulation of secondary injury mechanisms by microRNAs, a group of regulatory non-coding small RNA molecules following experimental traumatic brain injury (TBI); our recent data suggest that injury-induced changes in specific microRNAs are key to the activation of neuronal cell death pathways and ultimately to neuronal cell loss after TBI; and 2) the transcriptomic changes and their epigenetic underpinning that drive the molecular and cellular secondary injury processes.
Please see Dr. Stocia's faculty profile for a complete list of his publications.
You can also see his publications on PubMed.
Contact:
Email: Bstoica@som.umaryland.edu
Phone: (410) 706 5186
