Projects
New roles of oxidized phospholipids in modulation of septic inflammation, coagulopathy, traumatic injury and aging-related exacerbation of lung injury
Pioneering studies by Birukov group revealed previously unrecognized barrier enhancing properties of certain products of cell membrane phospholipid oxidation and proposed their utilization in vivo as new strategies for treatment of acute lung injury, inflammation and associated increased vascular leak syndromes. Current studies are focused on the development of a new group of synthetic cleavage-resistant phospholipid compounds as prototypes for future treatments of lung inflammation and barrier dysfunction. This project may uncover novel, previously unexpected mechanisms accelerating ALI recovery by oxidized phospholipids and may lead to the discovery of a new group of pharmacological molecules for the treatment of ALI, ARDS, and other diseases associated with increased vascular leakage and inflammation.
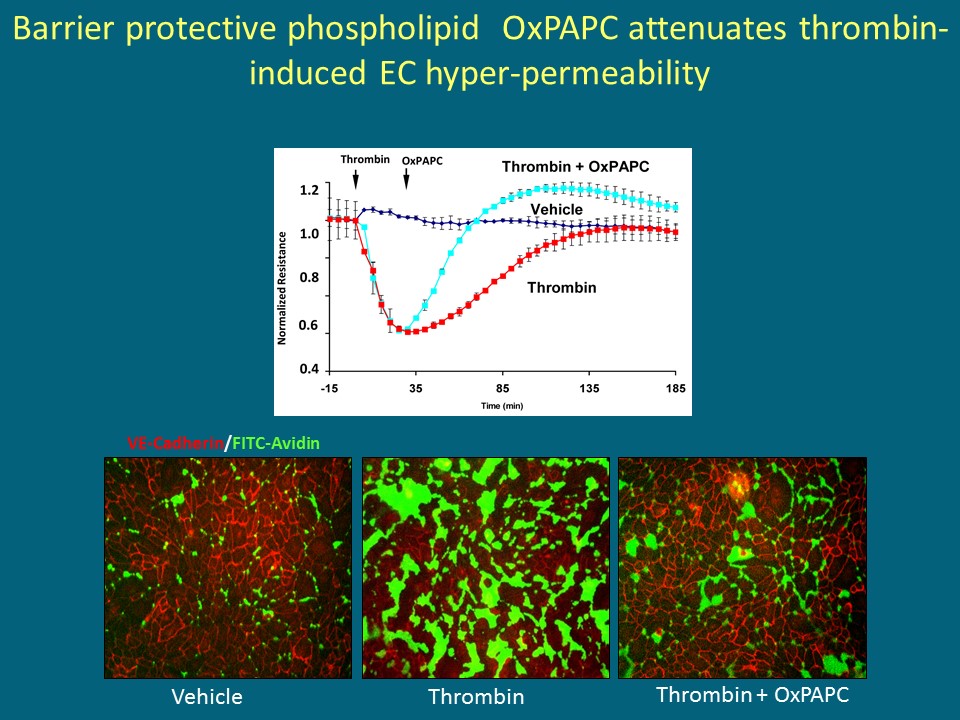 Fig 1: Barrier-protective oxidized phospholipids accelerate endothelial barrier recovery
Fig 1: Barrier-protective oxidized phospholipids accelerate endothelial barrier recovery
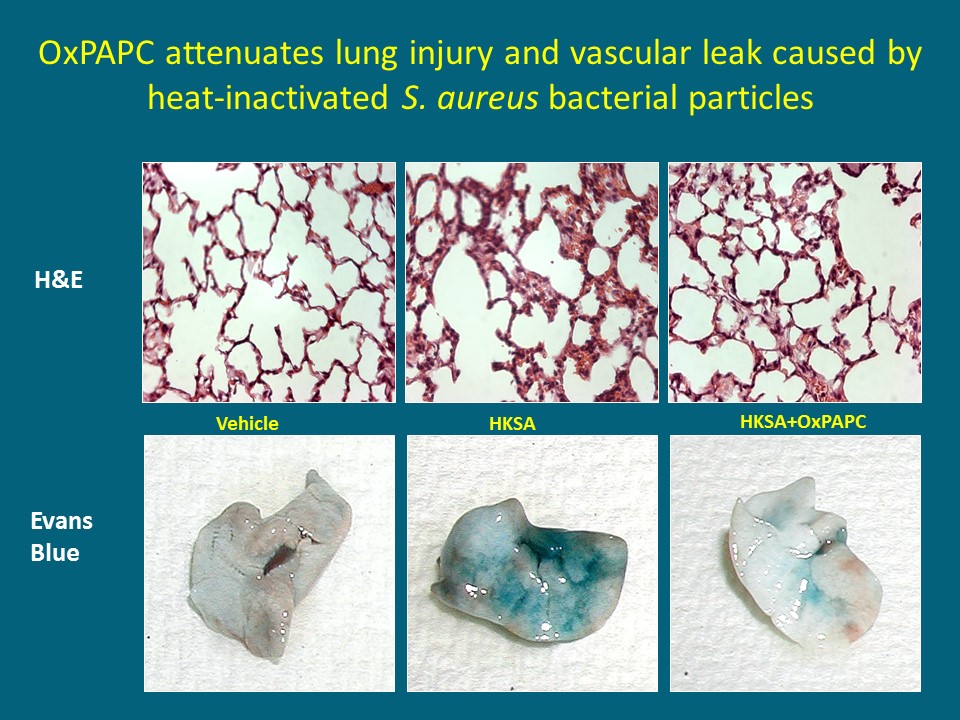 Fig. 2: Barrier-protective oxidized phospholipids attenuate septic lung inflammation and pulmonary edema
Fig. 2: Barrier-protective oxidized phospholipids attenuate septic lung inflammation and pulmonary edema
Mechanochemical regulation of vascular permeability and inflammation; role of pathologic mechanical stretch and substrate stiffness in endothelial pathobiology.
We continue characterization of endothelial mechanosensory complexes which mediate stretch magnitude-dependent barrier protective or barrier disruptive endothelial response. The proposed studies are aimed at understanding of the nature of magnitude-dependent regulation of Rac, Rap and Rho small GTPase signaling and delineation of key mechanosensitive regulatory proteins at focal adhesions, adherens junctions or Rho/Rac-specific guanine nucleotide exchange factors involved in magnitude dependent cell responses. These studies utilize state-of-the-art approaches such as cell cultures on elastic substrates of defined stiffness; a novel assay (XPerT) for the imaging of local permeability in CS-stimulated EC monolayers at 0.1-1 micron-range resolution recently developed by our group and analysis of cellular force distribution by traction force microscopy (TFM) and local changes in cellular signaling using FRET biosensors. These breakthrough technological developments will advance our understanding of the role of mechanical forces in endothelial biology and will enable a novel approach to high throughput screening of barrier protective compounds based on their effects on cell contractility in the physiologically relevant mechanical microenvironment.
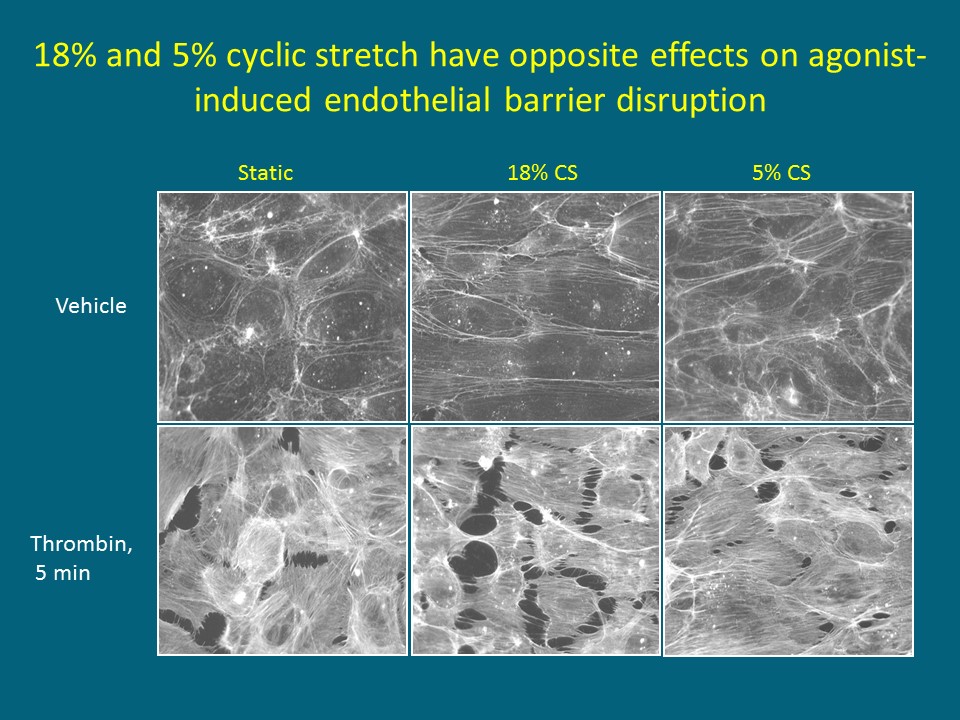 Fig. 3: Differential effects of physiologic and pathologic cyclic stretch on agonist-induced endothelial permeability
Fig. 3: Differential effects of physiologic and pathologic cyclic stretch on agonist-induced endothelial permeability
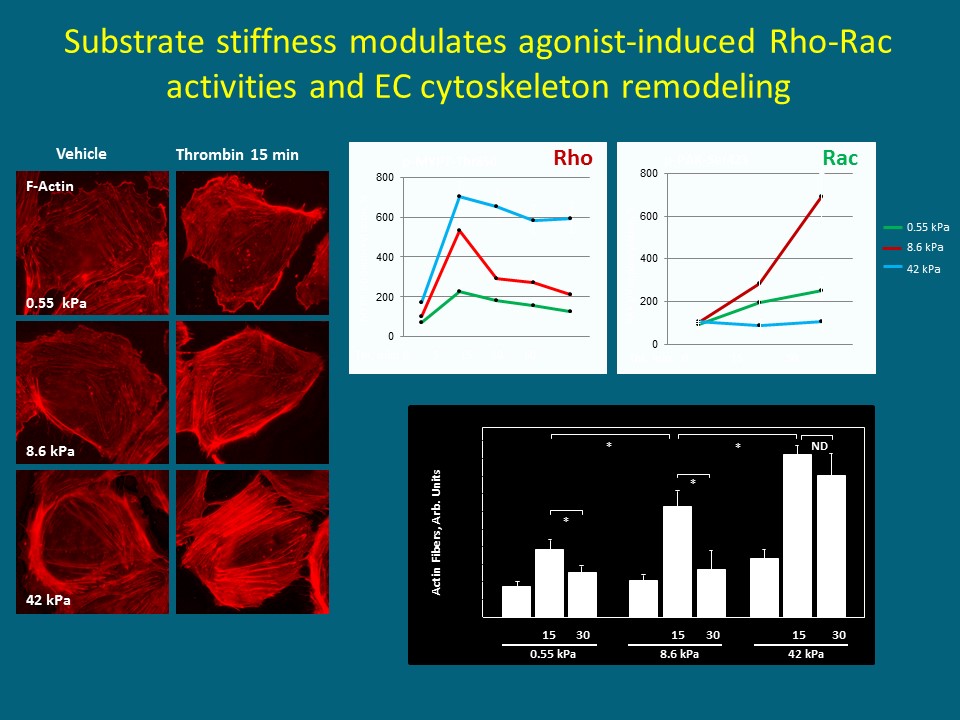
Fig. 4: Substrate stiffness is a major factor modulating endothelial responses to vasoactive and inflammatory molecules
Contact
Office: 410-706-2578
Email: kbirukov@som.umaryland.edu
