Education and Training for Clinical Investigators
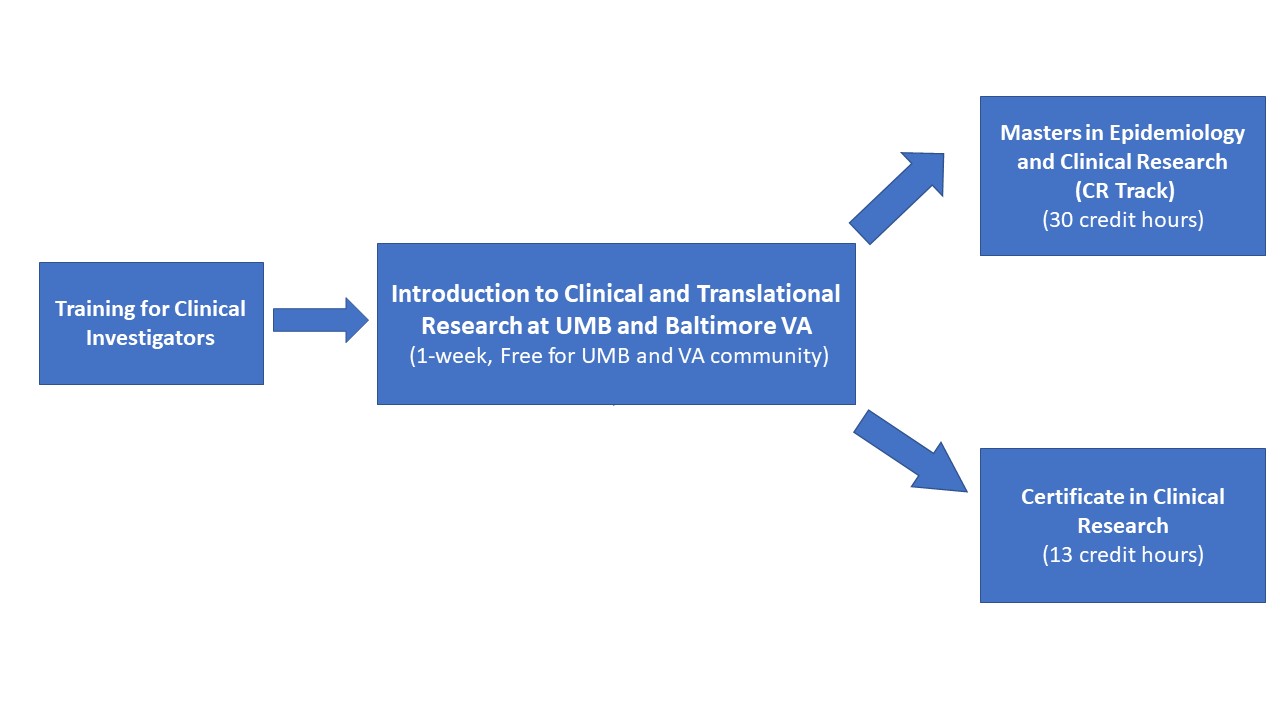
The goal of this program is to provide education and training in clinical and translational research for clinicians and scientists. This program includes a Master of Science degree in Epidemiology and Clinical Research (Clinical Research track) and a Certificate in Clinical Research.
The intensity of formal training can vary from a certificate program (13 credits) to the MS degree (30 credits), depending on the background and needs of the student.
Those enrolled in formal training programs will demonstrate the following knowledge, skills and attitudes:
- Critically appraise scientific literature.
- Formulate research questions and testable hypotheses.
- Operationalize dependent and independent variables.
- Design and conduct clinical studies that minimize bias and confounding.
- Respect and protect the rights and welfare of individuals participating in research.
- Know when and how to use appropriate statistical methods and interpret the results.
- Choose the appropriate sample size and power to test hypotheses.
- Effectively collect and maintain study data.
- Write a research grant proposal that will successfully compete for funding.
- Evaluate and communicate study findings to scientific and lay audiences.
- Manage a research team and build multidisciplinary collaborations.
Eligibility
Admission to the degree-granting and certificate programs is based on the criteria of the sponsoring program and the Graduate School.
Advisement
Students accepted to the MS or Certificate in Clinical Research program will be assigned an advisor. If you are unsure of which program is most appropriate, please read the Frequently Asked Questions and contact Jennifer Albrecht, PhD, at jalbrecht@som.umaryland.edu.
