The Hong Laboratory
Welcome
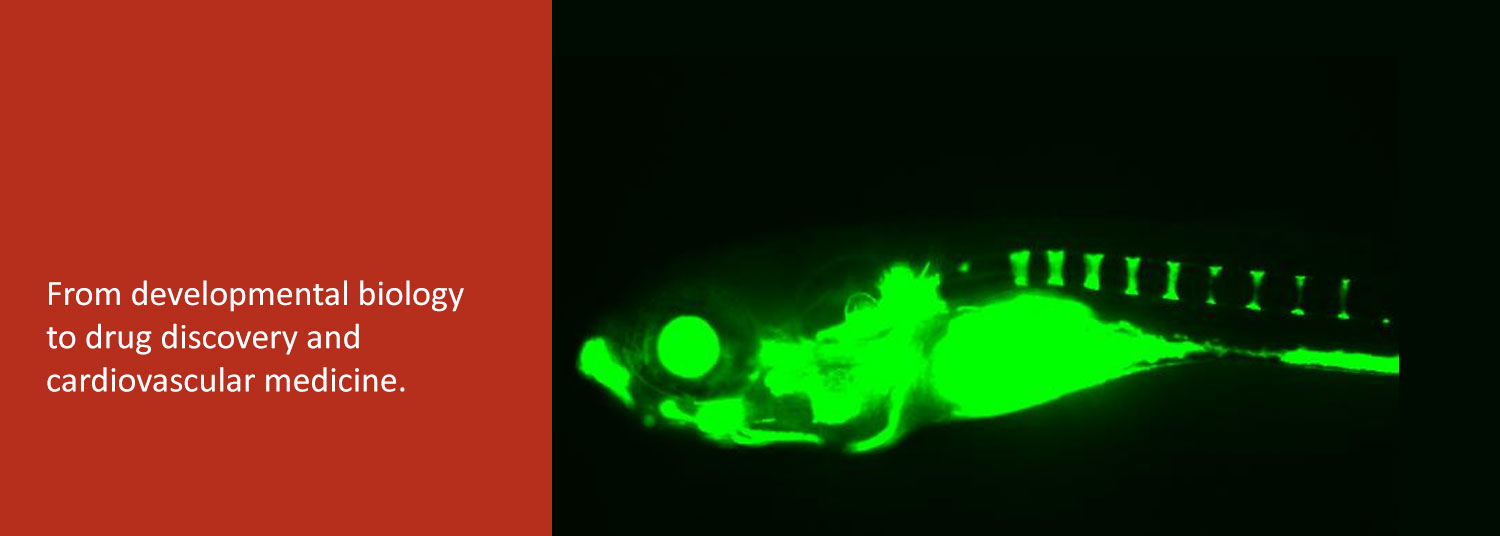
We function at the intersection of a variety of disciplines which include zebrafish development, drug discovery, and cardiovascular biology.
Our research can be divided into 2 broad areas. The first area involves chemical biology of vertebrate development, which entails discovery of small molecules that selectively modulate signaling pathways involved in embryogenesis. We have thus far discovered potent and highly selective chemical modifiers of bone morphogenetic protein (BMP), Wnt, Hedgehog, and lipid signaling pathways, among others.
Several of our compounds are first-in-class molecules with substantial therapeutic potential in rare and common diseases, including heterotopic ossification, cancers, atherosclerosis and heart failure. Thus, our chemical biological exploration is leading to new opportunities for innovative therapeutic programs.
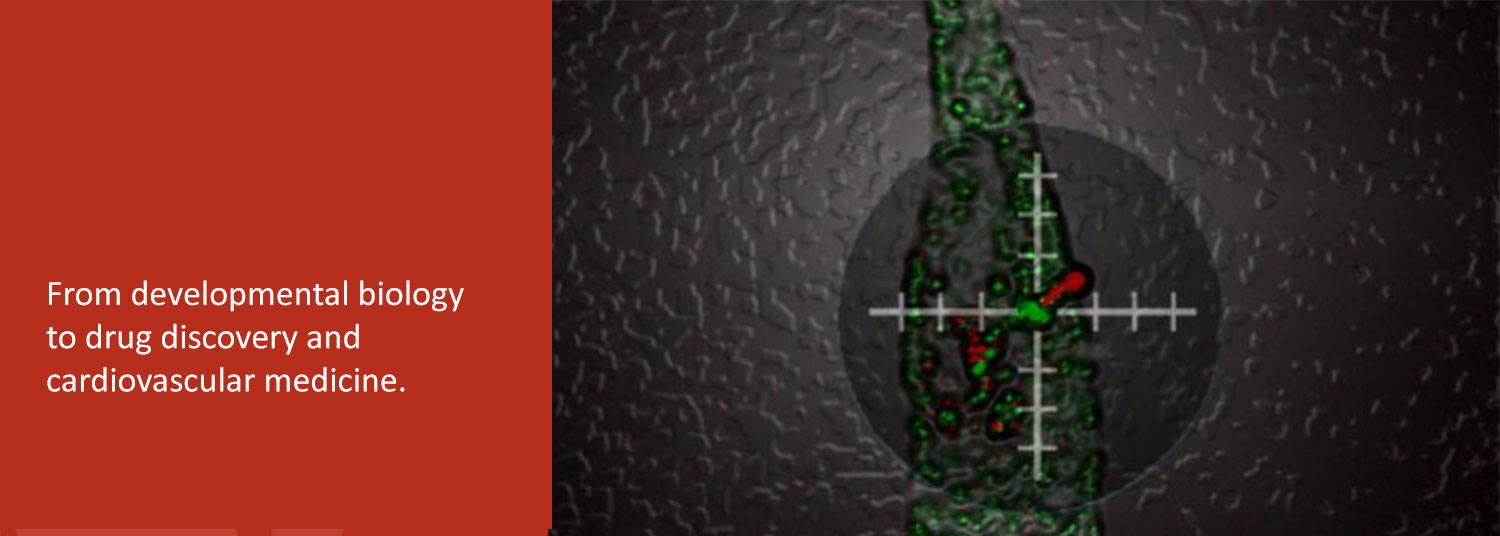
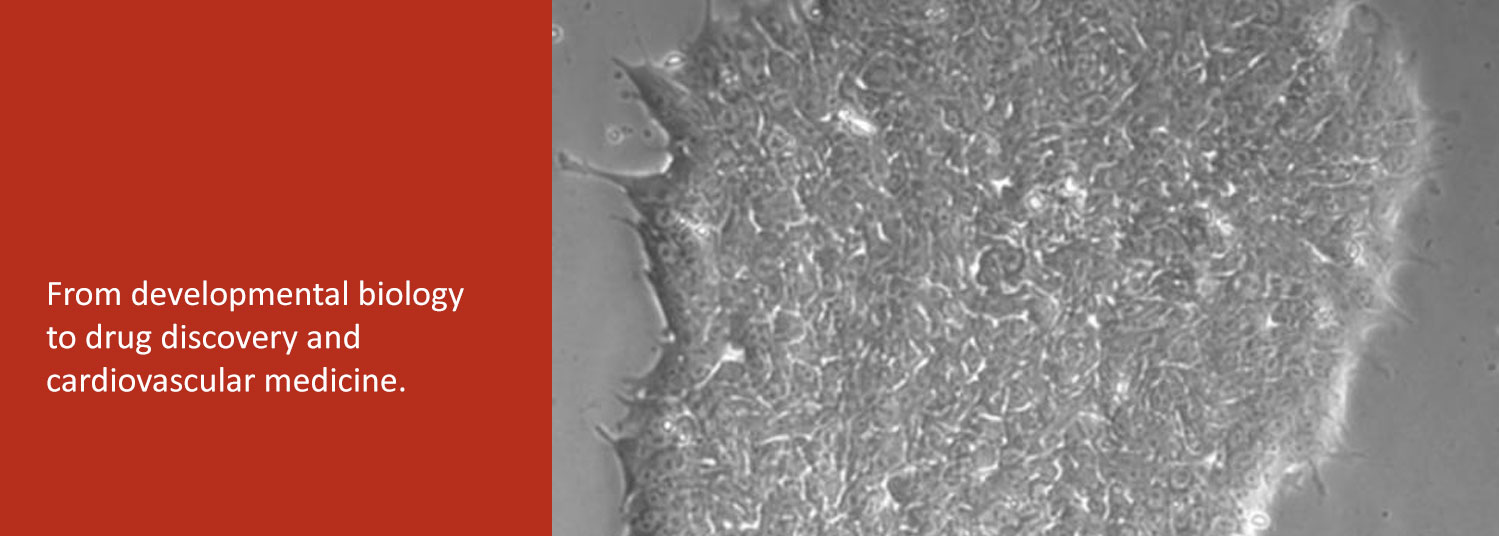
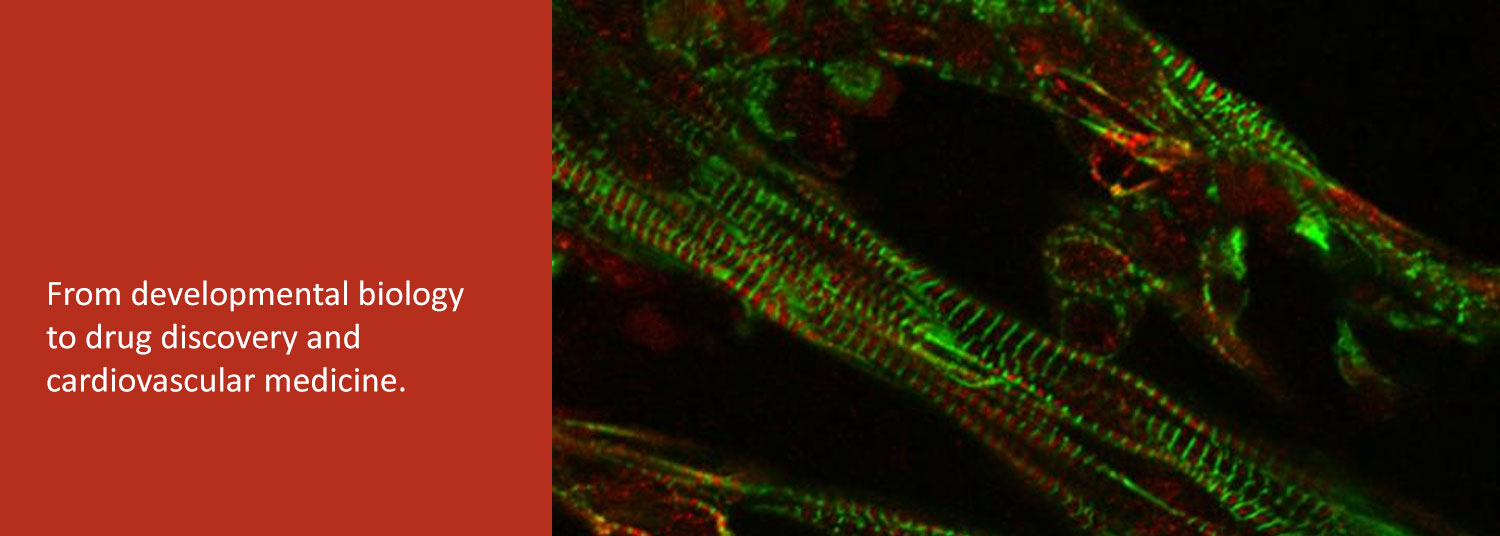
In the second, we are exploring the potential of patient-derived induced pluripotent stem cells (iPSC) to study and treat human heart diseases. We use human iPSCs as renewable cell sources for examining the fundamental cell biology and physiology of normal and diseased human cardiomyocytes.
Finally, we collaborate with bioengineers to develop human iPSC-derived heart tissues as a platform for drug discovery and evaluation.
Selected Achievements
2006 - Discovered the role of PI3K-ERK crosstalk in vascular development.
2008 - Discovered the first pharmacological inhibitor of the BMP pathway.
2010 - First large scale in vivo structure activity relationship (SAR) study outside the antimicrobial field.
2008, 2010 - First reported use of pharmacological inhibitors of BMP and Wnt pathways to induce cardiomyogenesis in pluripotent stem cells.
2012 - First description of the "dual chemical inhibition" for neural induction, which has become the most widely adopted method to generate human neurons from induced pluripotent stem cells.
2015 - Discovered phosphodiesterase-4 as a pharmacological target for hedgehog signaling inhibition.
2021 - With Dr. Jim Perry, showed that baseline low HDL was a risk factor for severe COVID-19 infection.
2022 - With Drs. Scott Devine and Jim Perry, discovered over 100K active mobile element insertions in 58K individuals, with implications for human disease such as cancer.
2023 - Discovered that mutations in RTTN gene cause infantile dilated cardiomyopathy by impairing programmed centrosome reorganization during cardiomyocyte maturation, revealing a window to potential cure for this rare but devastating childhood disease. The first time a defect in centrosome reorganization has been found to cause human disease.