Academic Title:
Professor
Primary Appointment:
Orthopaedics
Secondary Appointment(s):
Physiology
Administrative Title:
Division Head of Musculoskeletal Science
Phone (Primary):
(410) 706-2494
Fax:
(410) 706-0028
Education and Training
Education
1990-1994: B.S., Molecular and Cell Biology, The Pennsylvania State University
1996-2000: Ph.D., Biochemistry, Microbiology and Molecular Biology,The Pennsylvania State University
Post-Doctoral Training
2001-2004: Post Doctoral fellow in the lab of Roberto Civitelli, Washington University in St. Louis
Employment
2004-2013: Assistant Professor, University of Maryland School of Medicine
2013-2020: Associate Professor, Tenured, University of Maryland School of Medicine
2013-2020: Professor, Tenured, University of Maryland School of Medicine
Biosketch
Joseph P. Stains, PhD, is a Professor in the Department of Orthopaedics with a secondary appointment in the Department of Physiology at University of Maryland School of Medicine. He also has membership in the University of Maryland School of Medicine Center for Research on Aging, Center for Stem Cell Biology and Regenerative Medicine, and Center for Research on Biomedical Engineering and Technology.
Dr. Stains graduated from the Pennsylvania State University with a Bachelor of Science in Molecular & Cell Biology and, subsequently, a Ph.D. in Biochemistry, Microbiology, and Molecular Biology from this same institution. From 2001 to 2004, he was a post-doctoral fellow at Washington University in St. Louis in the Division of Bone & Mineral Diseases. In 2004, Dr. Stains was recruited to the University of Maryland School of Medicine as an Assistant Professor.
Dr. Stains' lab examines signaling and gene transcription in cells of the skeletal system, and how signaling networks affect osteogenic differentiation, bone formation and bone quality. Using a molecular level perspective, the lab examines cell-to-cell communication among bone cells and how intercellular communication coordinate new bone remodling and repair. Additionally, the lab examines the role of the cytoskeleton in mechanical load induced signaling in osteoblasts and osteocytes and how this mechano-signaling pathway can regulate the bioavailability of sclerostin, a key protein regulating bone homeostasis.
Dr. Stains has published more than 65 peer reviewed articles, 4 book chapters and has been consistently funded, including by the National Institutes of Health, Department of Defense, the Maryland Stem Cell Research Fund. He is active in the American Society of Bone and Mineral Research, where he served as a member of the finance committee, and is on the editorial board for the societies’ two journals, the Journal of Bone and Mineral Research and JBMR Plus. In 2020, he was named a Fellow of the American Society of Bone and Mineral Research. In addition, he is a standing member of the Skeletal Biology Devlopment and Disease Study Section at the NIH has been a frequent ad hoc member of grant review study sections for the NIH, Arthritis Foundation, and VA.
Dr. Stains has been active at the University level serving on the Graduate Council, School of Medicine Council, Molecular Medicine Admissions Committee, as well as serving as the course director for GPLS 645: Cell & systems Physiology since 2014.
Dr. Stains was recognized as a University System of Maryland's PROMISE AGEP Outstanding Faculty Mentor (2015) and the Research Preceptor of the Year in the Department of Medical and Research Technology (2011). In 2017, he and his collaborators were awarded a U.S. Patent for a multi-functional fluid flow device to test the mechanical response of cells in culture and a provisional patent on targeting the cytoskeleton to improve bone mass. Several of the manuscripts originating from his lab have been recognized for content, access and citations.
Research/Clinical Keywords
Osteoblast, Osteocyte, Mesenchymal Stem Cells, Bone, Intercellular Communication, Gap Junction, Connexin 43, Signal Transduction, Protein Kinase C, ERK, Runx2, Osterix, Musculoskeletal Biology, Osteoarthritis, Cytoskeleton, Mechanotransduction, Intermediate Filaments, Microtubules, CaMKII, Sclerostin.
Highlighted Publications
Williams KM, Leser JM, Gould NR, Joca HC, Lyons JS, Khairallah RJ, Ward CW, Stains JP. (2020) TRPV4 Calcium Influx Controls Sclerostin Protein Loss Independent of Purinergic Calcium Oscillations. Bone 136:115356 [PMCID: In Process]
Choi JY, Lai JK, Xiong ZM, Ren M, Moorer MC, Stains JP, Cao K (2018) Diminished canonical β-catenin signaling during osteoblast differentiation contributes to osteopenia in progeria. J Bone Miner Res 33:2059-2070. Co-corresponding author. [PMCID In Process]
Lyons JS, Joca HC, Law RA, Williams KM, Kerr JP, Shi G, Khairallah RJ, Martin SS, Konstantopoulos K, Ward CW, Stains JP(2017) Microtubules tune mechanotransduction through NOX2 and TRPV4 to decrease sclerostin abundance in osteocytes. Sci. Signal. 10, eaan5748. [PMCID: PMC5858867]
Buo AM, Tomlinson RE, Eidelman ER, Chason M, Stains JP (2017) Connexin43 and Runx2 Interact to Affect Cortical Bone Geometry, Skeletal Development, and Osteoblast and Osteoclast Function. J Bone Miner Res 32:1727-1738.[PMCID: PMC5550348]
Moorer MC, Stains JP (2017) Connexin43 and the intercellular signaling network regulating skeletal remodeling. Curr Osteoporos Rep 15:24-31. [PMCID: PMC5332069]
Moorer MC, Hebert C, Tomlinson RE, Iyer SR, Chason M, Stains JP(2017) Defective signaling, osteoblastogenesis, and bone remodeling in a mouse model of connexin43 C-terminal truncation. J Cell Sci 130:531-540. [PMCID: PMC5312734]
Moorer MC, Buo AM, Garcia-Pelagio K, Stains JP, Bloch RJ (2016) Deficiency of the intermediate filament synemin reduces bone mass in vivo. Am J Physiol Cell Physiol 311:C839-C845. [PMCID: In process]
Lyons JS, Iyer SR, Lovering RM, Ward CW, Stains JP (2016) Novel multi-functional fluid flow device for studying cellular mechanotransduction. J Biomech 49:4173-4179 [PMCID: PMC5164981].
Stains JP, Civitelli R (2016) A Functional Assay to Assess Connexin 43-Mediated Cell-to-Cell Communication of Second Messengers in Cultured Bone Cells. Methods Mol Biol, Vol 1437: 193-201 [PMCID: PMC4959905].
Gupta A, Anderson H, Buo AM, Moorer MC, Ren M, Stains JP (2016) Communication of cAMP by connexin43 gap junctions regulates osteoblast signaling and gene expression. Cell Signal [PMCID: PMC4899183]
Liu S, Niger C, Koh EY, Stains JP (2015) Connexin43 mediated delivery of ADAMTS5 targeting siRNAs from mesenchymal stem cells to synovial fibroblasts. Plos One 10:e0129999. [PMCID: PMC4468185].
Buo AM, Stains JP (2014) Gap junctional regulation of signal transduction in bone cells. FEBS Lett 588:1315-1321. PMCID: PMC3989400
Gupta A, Niger C, Buo AM, Eidelman, ER, Chen RJ, Stains JP (2014) Connexin43 enhances the expression of osteoarthritis-associated genes in synovial fibroblasts in culture. BMC Musculoskelet Disord, 15:425. PMCID: PMC4295231
Niger C, Luciotti MA, Buo AM, Hebert C, Ma V, Stains JP (2013) The Regulation of Runx2 by FGF2 and Connexin43 Requires the Inositol Polyphosphate/Protein Kinase Cð Cascade. J Bone Miner Res, 28:1468-1477. PMCID: PMC3657330
Hebert C, Stains JP (2013) An Intact Connexin43 is Required to Enhance Signaling and Gene Expression in Osteoblast-like Cells. J Cell Biochem, 114:2542-2550. PMCID: PMC3963279
Niger C, Buo AM, Hebert C, Duggan BT, Williams MS, Stains JP (2012) ERK acts in parallel to PKC delta to mediate the Cx43-dependent potentiation of Runx2 activity by FGF2 in MC3T3 osteoblasts. Am J Physiol Cell Physiol 302:C-1035-1044. PMCID: PMC3330735
Niger C, Lima F, Yoo DJ, Gupta RR, Buo AM, Hebert C, Stains JP (2011) The Transcriptional Activity of Osterix Requires the Recruitment of Sp1 to the Osteocalcin Proximal Promoter. Bone 49:683-692. PMCID: PMC3170016
Gupta RR, Yoo DJ, Hebert C, Niger C, Stains JP (2010) Induction of an osteocyte-like phenotype by fibroblast growth factor-2. Biochem Biophys Res Commun, 402:258-264. PMCID: PMC2993102
Niger C, Hebert C, Stains JP (2010) Interaction of Connexin43 and Protein Kinase C-delta during FGF2 Signaling. BMC Biochem, 11:14. PMCID: PMC2855512
Niger C, Howell FD, Stains JP (2010) Interleukin-1β increases gap junctional communication among synovial fibroblasts via the extracellular signal regulated kinase pathway. Biol Cell, 102:37-49. PMCID: PMC2874634
Lima F, Niger C, Hebert C, Stains JP (2009) Connexin43 potentiates osteoblast responsiveness to fibroblast growth factor 2 via a protein kinase C-delta/Runx2-dependent mechanism. Mol Biol Cell 20:2697-2708. PMCID: PMC2688549
For a complete, up to date list of publications from the Stains lab, click here.
Research Interests
The research in my lab examines signaling and gene transcription in cells of the skeletal system, and how signaling networks affect osteogenic differentiation, bone formation and bone quality.
GAP JUNCTIONS IN BONE
A major project in my lab explores the molecular details of how osteogenic cells use cell-to-cell signaling through connexin43-containing gap junctions to coordinate function using in vitro and in vivo models.
Gap junctions are intercellular channels formed by hexamers of connexins in one cell that dock with a hexameric array of connexins on an adjacent cell, forming an aqueous pore between the two cells. Gap junctions permit the direct intercellular exchange of small molecules, such as ions, metabolites and second messengers.
In bone, osteoblasts and osteocytes are highly interconnected via gap junctions composed primarily of connexin43. In these cells, connexin43 has been shown to play an important role in transmitting hormonal-, mechanical load- and growth factor-induced signals and, ultimately, in bone mass acquisition. In addition, mutations in Gja1, the gene encoding Cx43, result in the pleiotropic disorder oculodentodigital dysplasia, which includes skeletal manifestations.
Despite the clear importance of connexin43 in skeletal function, key molecular details of how connexin43 regulates bone mass acquisition, osteoblast differentiation and osteoblast/osteocyte function are unknown. My work is focused on answering key questions, such as what are the identities of the second messengers communicated by connexin43 gap junctions, what are the targets of these second messengers, and how do they regulate osteoblast/osteocyte function?
In my lab, we use in vivo models, as well as molecular, biochemical and cell biological approaches to determine the role of connexin43 in bone and to elucidate the molecular mechanisms underpinning the impact of connexin43 on osteoblast gene transcription, signal transduction and bone formation.
OSTEOCYTE MECHANOTRASNDUCTION AND THE CYTOSKELETON
A recent focus of my lab has been on the role of cytoskeletal proteins in the mechanically induced response observed in bone cells following mechanical loading. Bone dynamically remodels to adapt to mechanical loads to maintain its structural integrity. Bone-embedded osteocytes that reside in the fluid-filled lacunar-canalicular system are central to skeletal mechanoresponsiveness. We have been intersted in how these cells sense and respond to these mechanical cues to orchestrate bone remodeling and repair.
We have recently mechanistically linked fluid shear stress induced Ca2+influx to the degradation of sclerostin, a key regulator of bone formation. We have defined a specific subset of post-translationally modified microtubules that serve as rheostats, controlling the stiffness of the cytoskeleton and tuning the cells response to mechanical cues. Specifically, we have defined a mechanically activated NOX2-ROS-TRPV4-Ca2+-CaMKII pathway that leads to the rapid degradation of sclerostin protein.
During this work, we developed and patented a novel device to interrogate cell responses to fluid shear stress by both cell imaging and molecular techniques in the same device. Additionally, we collaborated on an investigated the role of the cytoskeletal regulator MARK3 on bone and examined the contribution of the multifunctional intermediate filament protein, synemin, to bone mass.
Gap Junctions
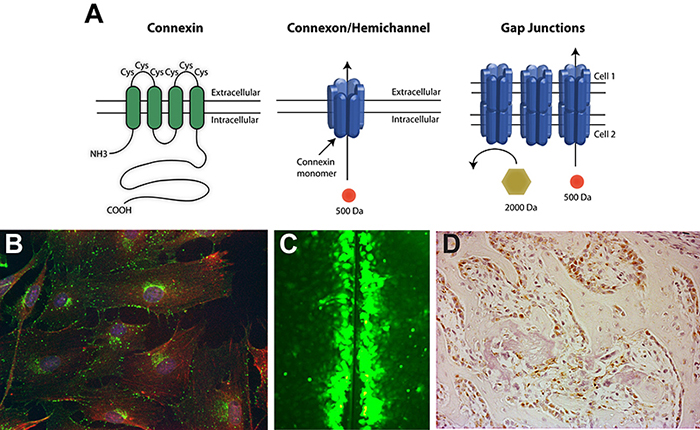
(A) Gap junctions are composed of connexin monomers. Six connexin monomers assemble to form a connexon or hemichannel in the plasma membrane of one cell. A hemichannel can then dock with a hemichannel on an adjacent cell to form a permeable channel between the cells. (B) Immunofluorescence of the gap junction protein connexin43 in osteoblast cells. Connexin43, green; actin, red; nuclei, blue. (C) Gap junctional communication (the exchange of low molecular weight molecules) between osteoblasts can be visualized by the scrape loading dye transfer assay. (D) Immunohistochemistry of connexin43 (reddish-brown) in osteoblasts during bone formation.
Grants and Contracts
Current Research Support:
03/01/2013-2/28/2025 "Spatial Control of Bone Remodeling by Gap Junction-Communicated cAMP" NIH/NIAMS R01 AR063631. Role: PI
3/01/2018-2/28/2023 “Mechanisms of osteocyte mechano-signaling and sclerostin regulationl” NIH NIAMS R01 AR071614. Role: PI (MPI)
Completed Research Support:
4/01/2019-3/31/2020 "Microtubules and Mechanobiology in the Crosstalk of Bone and White Adipose Tissue" Mid-Atlantic Nutrition Obesity Research Center Pilot & Feasibility Grant
10/01/2016-9/30/2017 “h-MSC Based Delivery of siRNAs to Chondrocytes and Synovial Tissue in a Rat Model” Pilot and Feasibility (P&F) Grant from the Baltimore Center for Musculoskeletal Research. Role Co-I
10/01/2015-9/30/2016 “Microtubule Dependent Mechanotransduction in Bone” Pilot and Feasibility (P&F) Grant from the Baltimore Center for Musculoskeletal Research. Role Co-I
07/01/2013-06/30/2016 "Optimal Treatment of Malignant Long Bone Fracture: Influence of Method of Repair and External Beam Irradiation on the Pathway and Efficacy of Fracture Healing" DoD PR120168. Role: Co-I
07/01/2014-06/30/2016 "The Role of the beta-Catenin Signaling Cascade in the Skeletal Phenotype of Hutchinson Gilford Progeria Syndrome" TEDCO/Maryland Stem Cell Research Fund, 2014-MSCRFE-0645. Role; PI
7/01/2012-6/30/2014 "Using Gap Junctions to Enhance Stem Cell Therapies in Osteoarthritis" TEDCO/MSCRF 2012-MSCRFE-0154-00. Role: PI
5/01/2006-8/30/2011 "Intercellular Signaling in Bone" NIH/NIAMS R01 AR052719. Role: PI
Lab Techniques and Equipment
Real time PCR, luciferase reporter assays, western blotting, cell transfections, cell culture, chromatin immunoprecipitations (ChIP), fluorescence microscopy, immunohistochemistry, skeletal histology, bone histomorphometry, whole mount staining and micro computed tomography.
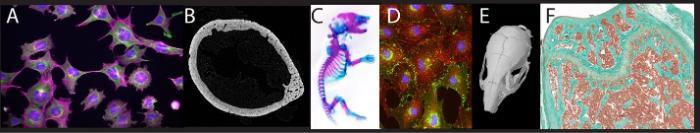
(A) Immunofluorescence of cytoskeleton proteins in osteocytes. (B) Sub-micron resolution microCT slice of a mouse femoral diaphysis. (C) Whole mount Alizarin Red/Alcian blue stained neonatal mouse. (D) Immunofluorescence of gap junctions (green) and actin (red) in cultured osteoblasts. (E) MicroCT scan of a mouse skull. (F) Trichrome stain of the mouse distal femur.
