Join the Fight 
For You, For Me, For All
Enrollment for this study has now closed. Daily questionnaires for enrolled participants will continue through October 31, 2021, after which only occasional longer surveys will be administered for the rest of 2021. We will continue to update the webpage with information for current participants. Publications from this study are highlighted below.
Thank you for your interest in learning more about this study. Researchers at the University of Maryland School of Medicine and University of Maryland Medical System (UMMS) partnered with the public to better understand the spread of COVID-19 in your community.
We recruited study participants to complete brief daily online questionnaires. The questionnaires were sent to participants by email or text message from Oracle, one of our study partners. The questionnaires asked participants about any symptoms they had related to COVID-19, possible exposures to COVID-19 and health care visits due to the disease. Participants also had the option to participate in antibody testing.
The study was available to any interested members of the general public aged 18 years and older, living in Maryland or the surrounding states.
Questions? Study staff are available to answer your questions about this research study and can be reached by phone at 443-457-3548 or via email at covid19-rx@som.umaryland.edu from 9am -5pm, Monday-Friday.
Participant Announcement
This research study is coming to a close and as of October 31, 2021, you will no longer receive the daily online questionnaire. You may still receive the occasional request from us to complete one of the additional longer surveys through to the end of 2021.
We wish to extend a huge THANK YOU to every single one of our participants. Without you this project would not have been possible! As we begin to finalize publications, we invite you to take a look at those which have already been published (see section below).
Raffle Winner Announcement
Thank you to all our participants in continuing the fight against COVID-19.
- For the week of June 1-7 the weekly raffle winners are from: Baltimore City (x4), Bel Air, Ellicott City, Glen Burnie, Havre de Grace (x2), Towson
- For the week of June 8-14 the weekly raffle winners are from: Baltimore city (x2), Finksburg, Annapolis, Kingsville, Waldorf, Ellicott City, Glen Burnie, Havre de Grace, Bel Air
- For the week of June 15-21 the weekly raffle winners are from:Pasadena, Baltimore City, Easton (x2), Severn, Sykesville, Jefferson, Manchester, Adelphi, Lutherville
- For the week of June 22-28 the winners are from Owings Mills, Severna Park, Baltimore City (2), Westminster, Columbia, Centreville, White Hall, Randallstown, Parkville
- For the week of June 29 - July 5 the winners are from Baltimore County (3), Baltimore City (2), Easton, Royal Oak, Clearwater Beach, Manchester, Chesapeake City
- For the week of July 6-12 the weekly raffle winners are from: Easton, Columbia, Chestertown, Glen Burnie, Linthicum Heights, Apple Valley, Laurel, Lancaster, Grasonville, Shrewsbury
- For the week of July 13-19 our winners are from: Baltimore City (3), Glen Burnie, Columbia (2), Bel Air, Towson, Centreville, and Hampstead.
- For the week of July 20-26 the weekly raffel winners are from: Baltimore (4), Chevy Chase, Joppa, Aberdeen, Ocean City, Middle River, University Park
- For the week of July 27-Aug 2 our winners are from: Brooklyn Park, Oxford, Parkton, Lutherville, Bel Air, Walkersville, Jarrettsville, Baltimore City, Reisterstown, La Plata
- For the week of Aug 3-9, our winners are from: LaPlata, Columbia, Cockeysville, Ellicott City, Bel Air, Towson, Baltimore City (2), Beltsville, Chestertown
- For the week of Aug 10-16, our winners are from: Bowie, Woodstock, Upper Marlboro, Arnold, Berwyn Heights, Church Hill, Timonium, Sykesville, Baltimore City, and Parkville
- For the week of Aug 17-23, our winners are from: Severn, Bel Air (2), Severna Park, Baltimore City (3), Sandy Spring, Forest Hill and Pasadena
- For the week of Aug 24-30, our winners are from: Upperco, Arnold, Towson, Bel Air (2), Abingdon, Baltimore City, Chestertown, Columbia, Forest Hill.
- For the week of Aug 31 - Sep 6, our were from: Annapolis (2), Centerville, Arnold, Laurel, Bridgeville, Harvre de Grace, Glen Burnie and Baltimore City (2)
- For the week of Sep 7-13, our winners are from: Glen Arm, Trappe, Baltimore City (2), Havre de Grace, Severna Park, Dunkirk, California, Goldsboro, Fallston.
- For the week of Sep 14-20, our winners are from: Baltimore City (3), Red Lion, Glen Arm, Parkville, Silver Spring, Hanover, Edgewood, Pasadena
- For the week of Sep 21-27, our winners are from: Abingdon, Baltimore City (3), Hurlock, Parkton, Ellicott City, Severna Park, Preston and Cockeysville.
- For the week of Sep 28 - Oct 4, our winners are from: Millersville, Arnold (2), Martinsburg, Timonium, Severn, Jessup, Columbia, Easton and Abingdon.
- For the week of Oct 5 - 11, our winners are from: Baltimore City (4), Ellicott City (2), Fallston, Pikesville, Manchester, and Pasadena.
- For the week of Oct 12 - 18, our winners are from: Reston, Queenstown, Centreville, Glen Burnie, Silver Spring, Arnold, Severna Park, Pasadena, Randallstown and Laurel.
- For the week of Oct 19 - 25, our winners are from: Bel Air, Brookeville, Laurel, Betterton, Chestertown, Rogersville, Greenbelt, Olney, Baltimore, Timonium.
- For the week of Oct 26 - Nov 1, our winners are from: Chestertown, Forest Hill, Ellicott City, Abingdon, Henderson, Havre De Grace, Baltimore City (2), Elkridge, Lutherville-Timonium.
- Our monthly winners for June are from: Edgewood, Hurlock, Stevensville, Linthicum
- Our monthly winners for July are from: Bel Air, Easton, Upperco, Owings Mills
- Our monthly winners for August are from: Parkton, Severna Park, Towson and Reisterstown
- Our monthly winners for September are from: Crownsville, San Diego, Washington DC, Windsor
- Our monthly winners for October are from: Bel Air, Baltimore City, Elkton, Hagerstown.
We wish to extend a special thank you and CONGRATULATIONS to everyone who completed their daily survey every day! Keep completing your daily survey to have a chance of winning in the next raffle draw!
COVID-19 Community Research Partnership Study Town Hall
University of Maryland School of Medicine and MedStar Health Research Institute hosted a virtual Town Hall on June 14th in celebration and gratitude for our study participants!
Over 400 people attended the Town Hall. During the event, study researchers provided study updates, answered participant questions about COVID-19, current safety practices, and the vaccine. Study participants also shared their experiences of being in the study.
If you missed this special event, you can watch the full program at https://youtu.be/DrlBJ8uB3n8. You can also view slides here: COVID-19 Community Research Partnership Town Hall Presentation June 2021
What is a research study?
Research studies are designed to gain scientific knowledge that may help people in the future.
What is the purpose and goal of this study?
As you are aware, the coronavirus pandemic is a public health emergency. By understanding the spread of the virus, who is at greatest risk and the conditions that put people at risk, researchers can help the medical community develop strategies to fight it. The goal of this study was to collect information about the community’s COVID-19 exposures, symptoms, and health care visits due to the disease. The study also collected information on the use of personal protective equipment for participants who were healthcare workers.
What have we learned from the study so far?
As the study comes to a close, we have turned our focus to analyzing the data and communicating our findings to other scientists and the general public. Please find the below links to all peer reviewed publications along with a brief summary of the key points.
Mini surveys were sent to participants after the Thanksgiving and Winter Holidays. These surveys collected information about behaviors during these holidays. The results showed that non-household members were present at 47% of Thanksgiving gatherings and 69% of Winter holiday gatherings. Most people did not fully follow recommended public health safety behaviors (mask wearing, staying outside, or getting a COVID-19 test prior) when attending holiday gatherings. These holiday gatherings and lack of following guidelines could have contributed to the well documented post-holiday surge in COVID-19 cases.
In December/January a survey was sent to participants asking about their intent to get the vaccine. Seventy-six percent of respondents indicated that they intended to get the vaccine. Based on reported intent to get vaccinated, the current vaccination rates as of May 15, 2021 are:
- Yes – 99% vaccinated
- No intent – 53% vaccinated
- Unsure – 80% vaccinated
- Preferred not to answer - 78% vaccinated
The most cited reason for not answering yes to the vaccine intent question was concerns over safety (although we note that COVID-19 vaccines have been proven to be safe in adults and teens in multiple large clinical trials and monitoring of people who received the vaccine after authorization). Among the CRP North Carolina participants, the initial responses to the survey did not correlate with their eventual vaccination rate.
Interpreting my at-home antibody test results
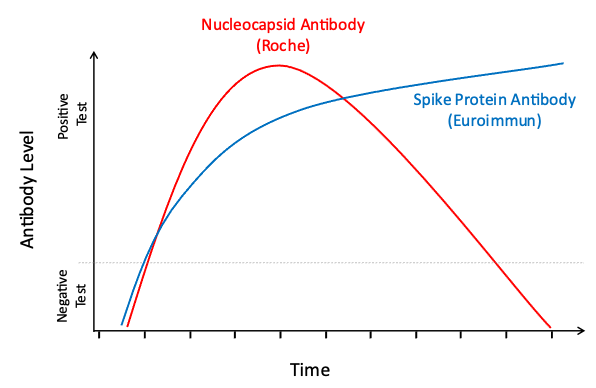
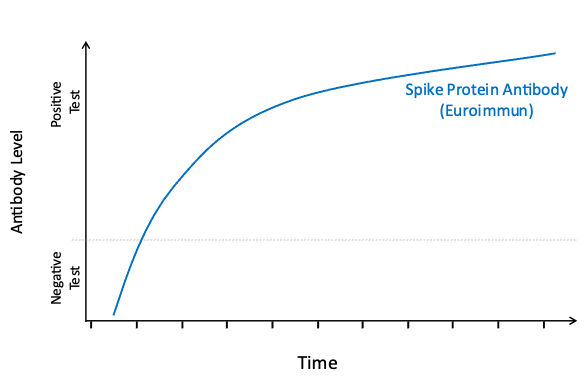
Your body will make different antibodies in response to natural SARS-CoV-2 infection than in response to COVID-19 vaccination. To better understand the spread of COVID-19 and the uptake of vaccination in the community, all participants who test positive for antibodies via EuroImmun LabCorp Testing are tested a second time using Roche LabCorp Testing to determine if their body’s response is due to exposure to the natural virus or vaccination. Interpretation of the results of these antibody tests can be found in the table below. Please note that the results of these tests should not be used to change your behavior. We encourage you to continue to follow guidance from the CDC and your physician concerning the best ways to prevent and manage COVID-19.
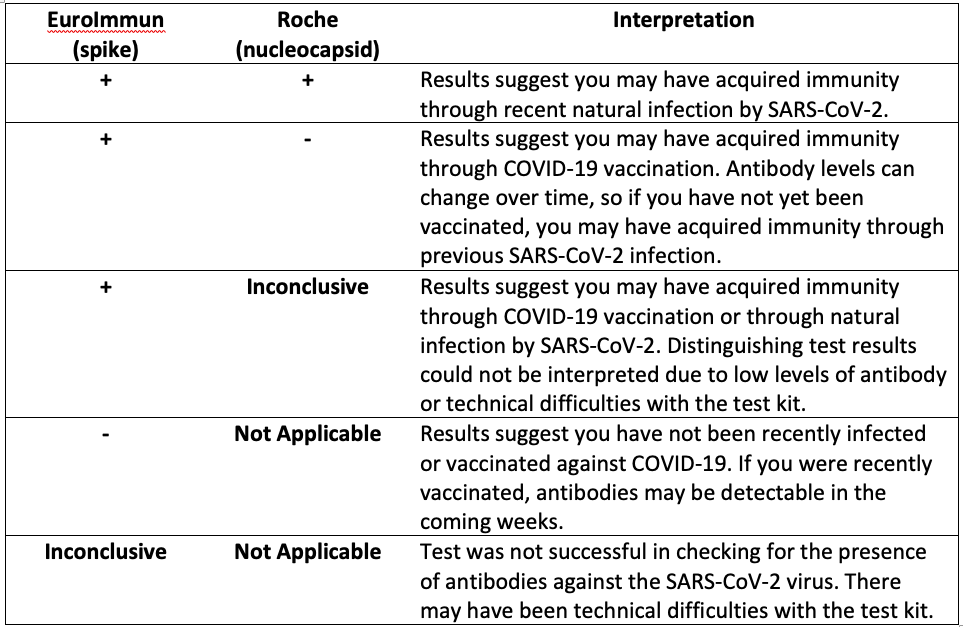
“-“ = negative test result“+” = positive test result
“Inconclusive” = the test result was not positive or negative. There may have been technical difficulties with the test kit.
“Not Applicable” = the Roche test (second test) is not performed if the EuroImmun (first test) test is negative or inconclusive.
EuroImmun: this test looks for spike protein antibody; spike protein is on the virus and is an important component of all COVID-19 vaccines.
Roche: this test looks for nucleocapsid antibody; nucleocapsid is not in any U.S. authorized COVID-19 vaccines.
Natural Infection
Vaccination
I cannot find the email reminder today. What happened?
Daily surveys will end on October 31, 2021. We will still send an occasional longer survey through the end of the year. If you believe you are missing an email reminder, please check your spam or junk folder. If you have checked your spam folder and still have not found an email, study staff are available to answer your questions about this research study and can be reached by phone at 443-457-3548 or via email at covid19-rx@som.umaryland.edu from 9am -5pm, Monday-Friday.
Who can answer my questions?
Questions? Study staff are available to answer your questions about this research study and can be reached by phone at 443-457-3548 or via email at covid19-rx@som.umaryland.edu from 9am -5pm, Monday-Friday.
What is the raffle?
We want to thank all our dedicated study participants and give you all the opportunity to win rewards by completing your daily survey updates!
We will be raffling off gift cards for study participants who complete their daily surveys. Here’s how it works:
- Every participant who completes their daily survey for 7 consecutive days is eligible to win a $10 gift card. We will select 10 winners every week!
- Every participant who completes their daily survey every day for the entire month is eligible to win a Grand Prize of a $50 gift card. We will select 4 winners every month!
Maximize your chances of winning by completing your daily survey every day. Any days missed due to Oracle outages will not be counted against your daily participation. You will be entered into the raffle prize draw every week and/or month that you are eligible.
Winners will be contacted via email or phone. Weekly winners will be notified each week. Monthly grand prize winners will be notified each month. The last raffles will occur the first week in November corresponding with the end of the daily surveys.
Who else is taking part in this study?
This study is part of the COVID-19 Community Research Partnership. We are working together with the following partners:
- WakeForest Baptist Health
- WakeMed
- Atrium Health
- New Hanover
- Vidant Health
- Campbell University
- MedStar Health
- University of Mississippi Medical Center
- New Orleans area health network – Tulane University site
Together we are working to fight COVID-19. Check out our national website at http://covid19communitystudy.org/
Who has enrolled?
Our goal was to enroll a representative sample of participants from Maryland and the surrounding states and in particular we wanted to enroll racial and ethnic minority groups as these groups have been seriously impacted by COVID-19. The data below reflects study participation since we began enrollment in late January 2021.
Study enrollment
More than 5,590 participants enrolled in the study and together they have completed more than 414,775 daily questionnaires.
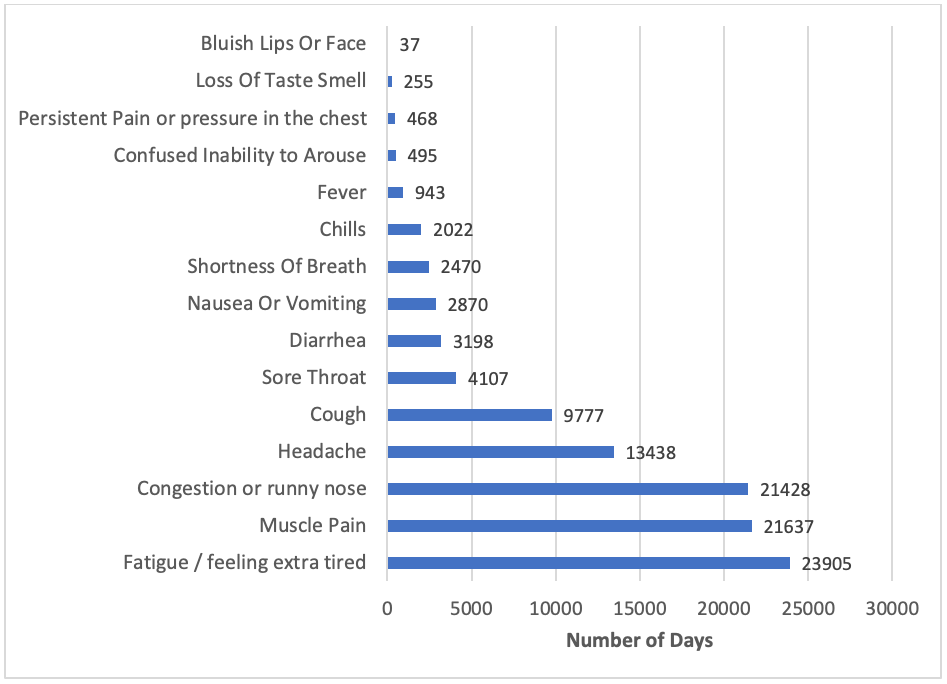
Most commonly reported symptoms
A total of 1,350 participants have reported experiencing at least one COVID-like symptom since their enrollment in the study. The most frequently reported symptoms so far have been fatigue, muscle pain, and congestion or runny nose. The graphic below shows all symptoms and the total number of days they have been reported.