Academic Title:
Professor
Primary Appointment:
Radiation Oncology
Administrative Title:
Director of Translational Radiation Sciences (DTRS) ; Associate Director of Basic Science in the UMGCCC.
Additional Title:
Director of Tumor Biology, Division of Translational Radiation Sciences, Department of Radiation Oncology, Co-Leader Program of Molecular and Structural Biology, Marlene and Stewart Greenebaum Comprehensive Cancer Center
Location:
Bressler Research Building, 10-037
Phone (Primary):
(410) 706-5105
Fax:
(410) 706-3260
Education and Training
University of Quebec at Trois-Rivières, Québec, Canada, B.Sc./Medical Biology, 1983
University of Montreal, M.Sc./ Clinical Sciences/Biochemistry, 1986
University of Montreal, Ph.D./Clinical Sciences/Biochemistry, 1988
Postdoctoral Fellow, Biotechnology Research Institute, National Research Council, Montreal Canada, Protein Engineering, 1988-1989
Guest Researcher, National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Maryland, Developmental Pharmacology, 1989-1991
Visiting Associate, National Cancer Institute, National Institutes of Health, Bethesda, Maryland, Laboratory of Molecular Pharmacology, 1991-1998
Biosketch
Dr. Carrier is an internationally renowned basic and translational cancer researcher, with specific training and expertise in genotoxic stress response and cancer progression. Her basic cancer research studies include activation of stress-responsive RNA binding proteins and their specific involvement in regulating protein translation in cancer cells. Her work has established hnRNP A18CIRP as a key regulator of protein translation in cancer cells by delineating its mechanism of action through specific signature motifs located in the 3’UTR of its targeted transcripts. Her research has continually been supported by different agencies including the National Institutes of Health and the VA.
Her translational cancer research includes her pioneered work on demonstrating the efficiency of a new class of anticancer drugs, histone deacetylase inhibitors, in combination with conventional anticancer drugs such as VP-16, ellipticine, doxorubicin, and cisplatin. This led to a recently completed Phase 1 clinical trial at her institution for relapsed and/or acute leukemia and myelodysplastic syndromes. She also actively contributes to the development of new clinical trials in radiation oncology, where her knowledge of the genotoxic stress response at the cellular and molecular levels complements new investigative clinical approaches with Low-Dose Fractionated Radiation Therapy (LDFRT) and radiation-stimulated immune response. Her work also involves chemopotentiation by LDFRT for disseminated abdominal cancers where she evaluates the role of Dual Oxigenase II as a potential biomarker for whole abdomen LDFRT. This work is currently funded by a VA merit award.
Dr. Carrier holds four patents, her publications have been cited over 7,000 times, including 13 papers with more than 100 citations each. She has lectured around the world, chair and co-chair numerous sessions at national and international cancer research meetings and served on the editorial boards of prestigious journals such as Cancer Research. Throughout her career, she has mentored a large number of talented scientists to pursue careers in the academics, government and industries related to cancer research.
Research/Clinical Keywords
Melanoma, gastric cancer, low dose radiation, histone deacetylase inhibitors
Highlighted Publications
Kim, M.S., Blake, M., Baek, J.H., Kohlhagen, G., Pommier, Y., and Carrier, F Inhibition of histone deacetylase increases cytotoxicity to anticancer drugs targeting DNA. Cancer Research, 63, 7291-7300, 2003. PMID: 14612526
Gojo, I,Tan, M, Fang, H-B, Sadowska, M., Lapidus, R., Baer, M.R., Carrier, F., Beumer,J.H., Anyang, B.N., Srivastava, R.K., Espinoza-Delgado, I.and Ross. D.D. Translational phase I trial of vorinostat combined with cytarabine and etoposide in patients with relapsed, refractory, or high- risk acute myeloid leukemia. Clinical Cancer Res. Apr 1;19(7):1838-1851. Epub 2013 Feb 12., 2013. PMID: 23403629
Diss, E, Nalabothula, N, Nguyen, DM, Chang, ET., Kwok, Y., Carrier, F. Vorinostatsaha promotes hyper-radiosensitivity in wild type p53 human glioblastoma cells. Journal of Clinical Oncology and Research, JSM Clin Oncol Res 2(1): 1004, 1-9, 2014. PMID: 25379568
Nguyen, D.M., Parekh, P.R., Chang, E.T., Sharma, N.K., Carrier, F. Contribution of Dual Oxidase 2 (DUOX2) to hyper radiosensitivity in human Gastric Cancer cells. Radiation Research, 184, 151–160, 2015. PMID: 26207686
Chang, E.T., Parekh, P.R., Yang, Q., Nguyen, D.M., and Carrier, F. The heterogenous ribonucleoprotein A18 (hnRNP A18) promotes tumor growth by increasing protein translation of selected transcripts in cancer cells. Oncotarget, (Vol.7) No 9, p. 10578-10593, 2016 Jan 25, Epub ahead of print. PMID: 26824423
Additional Publication Citations
Puga, A. Nebert, D.W., Carrier, F. Dioxin Induces Expression of c-fos and c-jun Proto-Oncoges and a Large Increase in Transcription Factor AP-1. DNA and Cell Biology, 11 (4), 269-281, 1992. PMID: 1605850.
Carrier, F., Owens, R.A., Nebert, D.W., Puga, A. Dioxin-Dependent Activation of Murine Cyp1a-1 Gene Transcription Requires Protein Kinase C- Dependent Phosphorylation. Mol. Cell. Biol., 12 (4), 1856-1863, 1992. PMID: 1312672.
Kastan, M., B., Zhan, Q., El-Deiry, W., S., Carrier, F., Jacks, T., Walsh, W., V., Plunkett, B., S., Vogelstein, B., Fornace, A.J.,Jr. A Mammalian cell cycle checkpoint pathway utilizing p53 and GADD 45 is defective in Ataxia Telangiectasia. Cell, 71: 587-597, 1992. PMID: 1423616.
Yarosh, D.B., Alas, L., Kibitel, J, O'Connor, A., Carrier, F., Fornace Jr, A.J., Cyclobutane pyrimidine dimers in UV-DNA induce release of soluble mediators that activate the Human Immunodeficiency Virus promoter. J. Invest. Dermatology l., 10, 1-5, 1993. PMID: 8388427.
Zhan, Q., Carrier, F., and Fornace, Jr., A. J. Induction of cellular p53 activity by DNA-damaging agents and growth arrest. Mol. Cell. Biol., 13, 4242-4250, 1993. PMID: 8321226
Carrier, F., Gatignol, A., Hollander, M.C., Jeang, K-T., and Fornace Jr., A.J. Induction of RNA-binding proteins in mammalian cells by DNA-damaging agents. Proc. Natl. Acad. Sci. USA, 91(4), 1554-1558, 1994. PMID: 7509078.
Zhan, Q., Lord, K.A., Alamo, I. Jr, Hollander, M.C., Carrier, F., Ron, D., Kohn, K.W., Hoffman, B., Liebermann, D. and Fornace, A.J. Jr. The gadd and MyD genes define a novel set of mammalian genes encoding acidic proteins that synergistically suppress cell growth. Mol. Cell. Biol., 14, 2361-2371, 1994. PMID: 8139541.
Carrier, F., McCary, J. M., Bae, I., Yarosh, D.B., and Fornace, Jr., A. J. Activation of HIV-1 Long Terminal Repeat by Ultraviolet light is serum and strain specific. AIDS Research and Human Retroviruses, 10, 767-773, 1994. PMID: 7986581.
Carrier, F., Chang, C-Y., Duh, J-L., Nebert, D.W., and Puga, A. Interaction of the regulatory domains of the murineCyp1a1 gene with two DNA binding proteins in addition to the Ah receptor and the Ah receptor nuclear translocator (ARNT). Biochemical Pharmacology , 48, 1767-1778, 1994. PMID: 9586958.
Carrier, F., Smith, M.L., Bae, I., Kilpatrick, K.E., Lansing, T.J., Chen, C-Y, Engelstein, M., Friend, S.H., Henner, H.D., Gilmer,T.M., Kastan, M.B., and Fornace, Jr., A.J. Characterization of Human Gadd45, a p53-regulated protein. J. Biol. Chem. 269 (51), 32672-32677, 1994. PMID: 7798274.
Kilpatrick, K.E., Carrier, F., Smith, M.L., Chen, C.-Y., Lee, A.J., Rusnak, D.W., Kastan, M.B., Fornace, A.J. Jr., Champion, B.R., Gilmer, T.M., and Su, J.L. The production and characterization of murine monoclonal antibodies to human Gadd45 raised against a recombinant protein. Hybridoma, 14 (4), 355-359, 1995. PMID: 8522347.
Carrier, F., Bae, I., Smith, M.L., Ayers, D.M., and Fornace, A.J., Jr. Characterization of the GADD45 response to ionizing radiation in WI-L2-NS cells, a p53 mutant cell line. Mutation Research, 352, 79-86, 1996. PMID: 8676920.
Sheikh, M.S., Carrier, F., Johnson, A.C., Ogdon, S.E., Fornace, Jr., A.J. Identification of an additional p53-responsive site in the human epidermal growth factor receptor gene promoter. Oncogene, 15, 1095-1101, 1997. PMID: 9285564.
Sheikh, M.S., Carrier, F., Papathanasiou, M.A., Hollander, M.C., Zhan, Q., Yu, K., and Fornace, Jr., A.J. Identification of several human homologs of hamster DNA damage inducible transcripts: cloning and characterization of a novel UV inducible cDNA that codes for a putative RNA binding protein. J.Biol.Chem., 272, 26720-26726, 1997. PMID: 9334257.
Carrier, F., Zhan, Q., Alamo, I., Hanaoka, F., and Fornace, Jr., A.J. Evidence for distinct kinase-mediated pathways in gadd genes responses. Biochemical Pharmacology, Vol. 55 No. 6, 853-861, 1998. PMID: 9586958.
Carrier, F., Georgel, P.T., Pourquier, P., Blake, M., Kontny,H.U., Antinore, M.J., Gariboldi, M., Myers, T. G, Weinstein, J.N., Pommier,Y,and Fornace, A.J., Jr. Gadd45, a p53-responsive stress protein, modifiesDNA accessibility on damaged chromatin. Mol. Cell. Biol. 19: 1673-1685,1999. PMID: 10022855
Zhan QM, Antinore MJ, Wang XW, Carrier F, Smith ML, Harris CC, Fornace AJ Association with Cdc2 and inhibition of Cdc2/cyclin B1 kinase activity by the p53-regulated protein Gadd45. ONCOGENE 18: (18) 2892-2900, 1999. PMID: 10362260.
Lin, J., Blake, M., Tang, C., Zimmer, D., Rustandi, R.R., Weber, D.J and Carrier, F. Inhibition of p53 transcriptional activity by the S100B calcium binding protein. J. Biol Chem. Sep 14;276(37):35037-35041, 2001. PMID: 11454863
Yang, C. and Carrier, F. The UV-inducible RNA binding protein A18 (A18 hnRNP) plays a protective role in the genotoxic stress-response. J.Biol.Chem., Dec 14: 276(50):47277-47284, 2001, PMID: 11574538.
Yang, C., Maiguel, D.A., and Carrier, F. Identification of Nucleolin and Nucleophosmin as genotoxic stress-responsive RNA binding proteins. Nucl.Acids Res., 30 (10):2251-2260, 2002. PMID: 12000845.
Kim, M.S., Blake, M., Baek, J.H., Kohlhagen, G., Pommier, Y., and Carrier, F. Inhibition of histone deacetylase increases cytotoxicity to anticancer drugs targeting DNA. Cancer Research, 63, 7291-7300, 2003, PMID: 14612526
Cha, H., Hancock, C., Dangi, S., Maiguel, D., Carrier, F., and Shapiro, P. Phosphorylation regulates nucleophosmin targeting to the centrosome during mitosis as detected by cross reactive phosphorylation specific MKK1/2 antibodies. Biochem.J, 378, 857-865, 2004. PMID: 14670079.
Maiguel, D.A., Jones, L., Chakravarty, D., Yang, C., and Carrier, F. Nucleophosmin sets a threshold for p53 response to UV radiation. Molecular and Cellular Biology, 24, 9, 3703-3711, 2004. PMID: 15082766
Lin, J., Yang, Q., Yan, Z., Markowitz, J.M., Wilder, P., Carrier, F and Weber, D.J. Inhibiting S100B restores p53 levels in primary malignant melanoma cancer cells.J.Biol.Chem. August 6: 279 (32), 34071-34077, 2004. PMID: 15178678
Markowitz, J., Chen, I., Gitti, R., Baldisseri, D.M., Pan, Y., Udan, R., Carrier, F., MacKerell, A.D., Jr., Weber, D.J. Identification and characterization of small molecule inhibitors of the calcium-dependent S100B-p53 tumor suppressor interaction. J. Med. Chem., 47, 5085-5093, 2004. PMID: 15456252
Kim, M.S., Baek, J.H., Chakravarty, D., Sidransky, D. and Carrier, F. Sensitization to UV-induced apoptosis by the histone deacetylase inhibitor Tricostatine A. Experimental Cell Research, 306, 94-102, 2005. PMID: 15878336
Markowitz, J., MacKerell, A.D., Jr., Carrier, F., Charpentier, T.H., Weber, D.J. Design of Inhibitors for S100B. Current Topics in Medicinal Chemistry, 5, 1093-1108, 2005. PMID: 16248785
Yang, R., Weber, D.J. and Carrier, F. Post-transcriptional regulation of thioredoxin by the stress-inducible hnRNP A18. Nucleic Acid Research, 34 (4), 1224-1236, 2006. PMID: 16513844
Wilder, P.T., Lin, J., Bair, C.L., Charpentier, T.H., Yang, D., Liriano, M., Varney, K.M., Lee, A., Oppenheim, A.B., Adhya, S., Carrier, F., Weber, D.J. Recognition of the tumor suppressor protein p53 and other protein targets by the calcium-binding protein S100B. Biochim Biophys Acta-Molecular Cell Research, 1763 (11):1284-1297 Sp. Nov 2006. PMID: 17010455.
Yang, C., Kim, MS, Chakravarty, D., Indig, F.E., Carrier, F. Nucleolin binds to the Proliferating Cell Nuclear Antigen and inhibits Nucleotide Excision Repair. Mol.Cell.Pharmacol.,1(3):130-137, 2009. PMID: 20336191
Yang, R., Zhan, M., Nalabothula, N., Yang, Q., Indig, F.E., Carrier, F. Functional significance for an heterogenous ribonucleoprotein A18 (hnRNP A18) signature RNA motif in the 3’UTR of Ataxia Telangiectasia Mutated and Rad3 related (ATR) transcript. J.Biol.Chem. March 19: 285 (12), 8887-8893, 2010. PMID: 20103595
Lin, J., Yang, Q., Wilder, P.T., Carrier, F (corresponding author), and Weber, D.J. The calcium-binding protein S100B down-regulates p53 and apoptosis in malignant melanoma. J.Biol.Chem, 2010. Aug 27;285(35):27487-98. PMID: 20587415
Nalabothula, N. Chakravarty, D.,Pierce, A. Carrier, F. Over expression of Nucleophosmin and Nucleolin contributes to the suboptimal activation of a G2/M checkpoint in Ataxia Telangiectasia fibroblasts. Mol.Cell.Pharmacol.,2(5), 179-189, 2010. PMID: 21499441
Abdelmohsen, K., Tominaga, K., Lee, E-K., Srikantan, S., Kang, M.J., Kim, M., Selimyan, R., Martindale, J.,Yang, X., Carrier, F., Zhan, M., Becker, K., Gorospe, M. Enhanced translation by Nucleolin via G-rich elements in coding and non-codingregions of target mRNAs. Nucleic Acids Res. 2011, Vol. 39, No. 19 8513–8530. PMID: 21737422
Indig, F.E., Rybanska, I., Karmakar, P., Devulapalli, C., Haiqing, F., Carrier, F. and Bohr, V.A. Nucleolin inhibits G4 oligonucleotide unwinding by Werner Helicase. PLoS One. 2012;7(6):e35229. Epub 2012 Jun 4. PMID: 22675465
Gojo, I,Tan, M, Fang, H-B, Sadowska, M., Lapidus, R., Baer, M.R., Carrier, F., Beumer,J.H., Anyang, B.N., Srivastava, R.K., Espinoza-Delgado, I.and Ross. D.D. Translational phase I trial of vorinostat combined with cytarabine and etoposide in patients with relapsed, refractory, or high-risk acute myeloid leukemia. Clinical Cancer Res. Apr 1;19(7):1838-1851. Epub 2013 Feb 12., 2013. PMID: 23403629
Diss, E, Nalabothula, N, Nguyen, DM, Chang, ET., Kwok, Y., Carrier, F. Vorinostatsaha promotes hyper-radiosensitivity in wild type p53 human glioblastoma cells. Journal of Clinical Oncology and Research, JSM Clin Oncol Res 2(1): 1004, 1-9, 2014. PMID: 25379568
Nguyen, D.M., Parekh, P.R., Chang, E.T., Sharma, N.K., Carrier, F. Contribution of Dual Oxidase 2 (DUOX2) to hyper radiosensitivity in human Gastric Cancer cells. Radiation Research, 184, 151–160, 2015. PMID: 26207686
Chang, E.T., Parekh, P.R., Yang, Q., Nguyen, D.M., and Carrier, F. The heterogenous ribonucleoprotein A18 (hnRNP A18) promotes tumor growth by increasing protein translation of selected transcripts in cancer cells. Oncotarget, (Vol.7) No 9, p. 10578-10593, 2016 Jan 25, Epub ahead of print. PMID: 26824423
Research Interests
My laboratory is interested in basic and translational cancer research. Our main focus is to understand molecular events underlying cancer progression. Our goal is to delineate intrinsic differences between normal and cancer cells in order to more specifically target cancer cells and improve current cancer therapies.
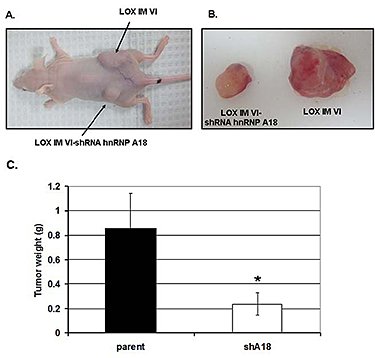
Carcinogenesis is a multiple steps process that includes initiation, promotion, transformation and finally propagation of the cancer cells. Our basic science projects focus on the first two steps of carcinogenesis, initiation and propagation and our collaborations on translational cancer research allow us to target the last step of carcinogenesis, propagation. The initiation step is usually triggered by exposure to carcinogens that can damage DNA. The cellular response that ensues, genotoxic stress response, is complex but generally plays a protective role against the cellular insults. Our studies on the genotoxic stress response have focused on the role of stress-activated RNA binding proteins. We have recently identified the stress-responsive heterogeneous ribonucleoprotein A18 (hnRNP A18) as a key regulator of protein translation in cancer cells (1). hnRNP A18 is usually not expressed in normal cells but is upregulated in about 20-30% of several human cancers including, breast, prostate, colon and melanoma. We have identified a consensus hnRNP A18 RNA binding motif in several mRNAs transcripts that can confer growth advantages to cancer cells (Fig.1). Down regulation of hnRNP A18 reduces tumor growth by 70-80% in a xenograft mouse model (Fig.2). Our long term goal is to identify and/or develop new drugs to rationally target hnRNP A18 in cancer cells in order to stop or control cancer progression. As a first step toward this goal we are collaborating with Drs. David Weber and Alex MacKerell to solve hnRNP A18 three dimensional structure and use Computer Aided Drug Design (CADD) to identify small molecules inhibitors.
<td ">
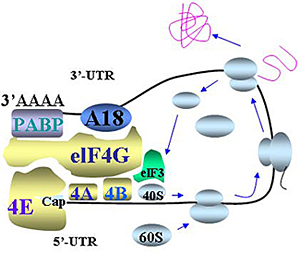
- Figure 1: mRNA circulation model of translational control involving hnRNP A18. hnRNP A18 may mediate this 5’UTR and 3’UTR loop formation by binding both the 3’UTR of transcripts and the initiation factor elF4G in the translation initiation complex. PABP: PolyA Binding Protein. The arrows indicate the direction of ribosome scanning and translating.
|
Propagation is the last and most devastating step of carcinogenesis. A major goal of several chemotherapeutic regimens is therefore to prevent or inhibit this step. The inhibitors of histone deacetylases (HDACIs) are considered one of the most promising anticancer drugs in development (2). As their name suggests, their primary targets are enzymes that deacetylate histones but several non-histone targets have also been described. HDACIs are most effective as combination agent against cancer cell proliferation. They are about ten times more efficient on cancer cells as compared to normal cells. The reason for this preferred sensitivity is not fully understood but is currently being investigated by a number of laboratories including ours. Our lab published one of the first studies demonstrating (3,4)that pre-treatment of cancer cells with HDACIs sensitize cancer cells to conventional chemotherapeutic agents. This work served as a basis to develop a recently completed Phase 1 clinical trial lead by Dr. Douglas Ross at the University of Maryland Greenebaum Cancer Center, to evaluate the effect of HDACI pretreatment in combination with cytarabine and etoposide in patients with relapsed, refractory, or high-risk acute myeloid leukemia (5). We are also interested in studying the effect of HDACIs and the alkylating agent temozolomide (TMZ) on promotion of hyper-radiosensitivity (HRS) in order to develop new clinical trials with low dose fractionated radiation therapy (LDFRT). Our preclinical data on HDACIs and TMZ demonstrated that HDACIs and TMZ can promote HRS in glioblastoma cells (6). These data served as a basis to develop a currently ongoing Phase II clinical trial (1224GCC) at our institution led by Dr. Young Kwok to evaluate the effect of Low-Dose Whole Brain Radiotherapy with Concurrent Temozolomide and Adjuvant Temozolomide in Patients with Newly-Diagnosed Glioblastoma Multiforme. We are currently pursuing similar studies on LDFRT to study the potential use of Low-Dose Fractionated Whole-Abdomen Radiation Therapy (LDFRT) as a chemosensitizer in patients with peritoneal carcinomatosis from gastric or gastroesophageal junction primary adenocarcinomas and to study LDFRT for colon cancer treatments.
Clinical Specialty Details
Translational Research
Awards and Affiliations
- 1990: International fellowship. Among the first awardees of a Long-term postdoctoral fellowship from the Human Frontier Science Program Organization.
- 1991: International fellowship. Visiting associate Fellowship from the National Institutes of Health
- 1994: Co-author on the second most-cited paper in biology in 1994. Science Watch, September 1994, p.5; Kastan, M., B., Zan, Q., El-Deiry, W., S., Carrier, F., Jacks, T., Walsh, W., V., Plunkett, B., S.,Vogelstein, B., Fornace, A.J.,Jr. A Mammalian cell cycle checkpoint pathway utilizing p53 and GADD 45 is defective in Ataxia Telangiectasia. Cell 71: 587-597
- 1992 1998, 1999: Intramural award entitled: "Induction of Mammalian RNA-Binding Proteins" from the office of the Dean, University of Maryland, School of Medicine
- 2001: Graduate Student Research Day Award, 2nd Place in Molecular Biology (Dony Maiguel)
- 2002: Sponsor: Brigid Leventhal Award from the American Association for Cancer Research (Myoung Sook Kim).
- 2002: Sponsor: Graduate Student Research Day Award, 2nd Place in Molecular Biology (Dony Maiguel)
- 2003: Sponsor: Graduate Student Research Day Award, 1st Place in Molecular Biology (Jing Lin)
- 2003: Sponsor: Graduate Student Research Day Award, 2nd Place in Molecular Biology (Dony Maiguel)
- 2004-present: Biography selected for publication in Who's Who in America
- 2004-present: Biography selected for publication in Who's Who in the World
- 2004-2007: National Kidney Foundation. Post-doctoral fellowship (Devulapalli Chakravarty) Editorial board: Cancer Research, J.Clinical Oncology and Research
- 2007-2009: Assistant Professor, Marlene and Stewart Greenebaum Cancer Center, University of Maryland, Baltimore.
- 2009-2014 : Associate Professor, Marlene and Stewart Greenebaum Cancer Center, Department of Radiation Oncology, University of Maryland, Baltimore.
- 2014: Tenured Associate Professor, Marlene and Stewart Greenebaum Cancer Center, Department of Radiation Oncology, University of Maryland, Baltimore.
- 2017-present Tenured Professor, Marlene and Stewart Greenebaum Cancer Center, Department of Radiation Oncology, University of Maryland, Baltimore.
- 2017-present: Research Health Scientist, VA Maryland Health Care System (VAMHCS), Research and Development Service
- 2018-present: Standing member NIH R01 DMP study section
- 2018-present: Director Tumor Biology, Division of Translational Radiation Sciences, Department of Radiation Oncology, School of Medicine, University of Maryland, Baltimore
- 2019-present Co-leader Molecular and Structural Biology Program, University of Marlyland, Marlene and Stewart Greenebaum Comprehensive Cancer Center
Grants and Contracts
Ongoing Research Support
I01BX003437 VA Merit Award Carrier (PI) 7/1/17-6/30/21
Title: Chemopotentiation by Low Dose Fractionated Radiation Therapy for disseminated intra-abdominal cancers
This project aims at determining the role of Dual Oxygenase II in mediating hyper radiosensitivity in gastric cancer cells and evaluate whether it could be used as a biomarker for cancer patients stratification for whole abdomen low dose radiation.
R25CA186872-01A1 NIH Hassel (PI) 09/01/15-08/31/20
Title: The Nathan Schnaper Intern Program in Translational Cancer Research
The overarching goal of this grant is to inspire and train the next generation of cancer researchers and physicians. Towards this goal, the grant provides funds to expand the educational, laboratory and clinical training components of this longstanding program. In addition, funds are provided for housing and transportation to successfully recruit a diverse and highly qualified pool of applicants from across the US.
1 P30 CA 134274-01 NIH Cullen (PI) 08/08/2006 – 07/31/2017
Title: University of Maryland Greenebaum Cancer Center Support Grant
This is an NCI Cancer Center Support Grant. The Cancer Center Support Grant (CCSG) provides the resources and infrastructure to facilitate the coordination of interdisciplinary programs across a broad spectrum of research from basic laboratory research to clinical investigation to population science.
T32CA15427406 NIH Antalis (PI) 07/01/16-06/30/21
Title: Training Grant in Cancer Biology
The Program in Cancer Biology launched in 2011 trains postdoctoral and predoctoral trainees for careers in cancer research. The program is based in the University of Maryland Marlene and Stewart Greenebaum Cancer Center (UMGCC), and includes UMGCC faculty from multiple basic science and clinical departments - the majority based on the University of Maryland Baltimore (UMB) campus, with others from the University of Maryland College Park (UMCP) and the University of Maryland Baltimore County (UMBC).
Professional Activity
- 2005, 2008: Invited grant reviewer for U.S. Civilian Research & Development Foundation (CRDF)
- 2008-present: NIH Grant reviewer: ARRA RC1 Challenge Grant applications. ZRG1 OTC-K (58) in Oncology-2 Translational Clinical IRG (OTC); ZRG1 OBT-B(02) study section: Cancer Biology and Therapy,Special Emphasis Panel focused on “Fellowship: Oncological Sciences” NIH (ZRG1 FO9B-P (20), Special Emphasis Panel to review applications in response to PAR-12-144, “NCI Small Grants Program for Cancer Research (RO3/R21), Tumor Cell Biology.
- 2005-present: Grant reviewer for a variety of other agencies: Association for International Cancer Research, United Kingdom, U.S. Civilian Research and Development Foundation (CRDF), National Aeronautics and Space Administration (NASA), National Science Foundation;
- 1990-present: Review manuscripts for Cancer Research, Carcinogenesis, Oncogene, Molecular and Cellular Biology, British Journal of Cancer, Molecular Cancer Therapeutics, Blood, Leukemia, FEBS Letters, Radiation Research, Cell Growth & Differentiation, Radiation Oncology, Environmental Health Perspectives, International Journal of Cancer, European Journal of Cancer, Experimental Cell Research.
- 2013-2016: Editorial Board of Cancer Research
- 2013-present: Editorial Board of Journal of Clinical Oncology and Research
- 2018-present: Standing member NIH R01 DMP study section