Academic Title:
Professor
Primary Appointment:
Neurobiology
Secondary Appointment(s):
BioChemistry&Molecular Biology
Additional Title:
Professor
Location:
HSF-II S267
Phone (Primary):
410-706-4778
Education and Training
- University of California at Berkeley, A.B. Biochemistry 1975
- University of Wisconsin at Madison, Ph.D., 12/1980
- Post-Doctorate, NIH (NIMH), Neuropharmacology, 1981-1984
Biosketch
The Lindberg lab works on the chaperone mechanisms used to maintain both the neuronal secretory pathway and the synaptic environment free of misfolded protein aggregates. They have discovered that two small chaperones, specifically expressed in neurons and endocrine cells, control the aggregation of a number of secretory proteins, including proteins known to be involved in neurodegeneration. The Lindberg lab would like to better understand the physiological mechanisms that these chaperones use to control aberrant protein assembly and aggregation within the secretory pathway –as well as within the synaptic cleft. Since most neurodegenerative diseases involve abnormal protein aggregation, this work is relevant to three such diseases: Parkinson's; Alzheimer's; and amyotrophic lateral sclerosis. By identifying natural mechanisms which act to block the progressive protein misfolding occurring both with aging and in disease, they hope to arrive at potential therapeutics that can be used to slow or halt proteostatic misfunction. Indeed one project has already shown a beneficial impact of chaperone overexpression on motor dysfunction in Parkinson’s disease.
Dr. Lindberg has been continuously funded by the National Institutes of Health since 1985, and has published 178 peer-reviewed papers, invited reviews, and book chapters, with about 8500 citations to date.
Research/Clinical Keywords
neurodegeneration, Alzheimer’s disease, Parkinson’s disease, protein chaperones, proteostais, ALS
Highlighted Publications
Lindberg, I., Shorter, J. Wiseman, R.L., Chiti, F., Dickey, C.A., and McLean, P.J. (2015) Chaperones in neurodegeneration. J. Neurosci., 35:13853-9. PMID: 26468185
Jarvela, T., Lam, H.A, Helwig, M., Lorenzen, N., Otzen, D.E., McLean, P.J., Maidment, N.T. and Lindberg, I. (2016) The neural chaperone proSAAS blocks alpha-synuclein fibrillation and neurotoxicity. Proc. Natl. Acad Sci. 113(32):E4708-15. PMID: 27457957 PMID:27457957
Ramos-Molina, B., Martin, M.G., and Lindberg, I. (2016) “PCSK1 variants and human obesity”, vol. Genetics of Monogenic and Syndromic Obesity, in series Progress in Molecular Biology and Translational Science. P. Michael Conn, Ed.; Academic Press. PMID:27288825
Winters, A.*, Ramos-Molina, B.*, Jarvela. T.S., Yerges-Armstrong, L., Pollin, T.I., and Lindberg, I. (2017) Functional analysis of PCSK2 coding variants: a founder effect in the Old Order Amish population. Diabetes Res. and Clinical Practice. 131:82-90 *Co-first authors. PMID:28719828
Katorcha, E., Makarava, N., Lee, Y.J., Lindberg, I., Monteiro, M.J., Kovacs, G.G., and Baskakov, I.V. (2017) Cross-seeding of prions by aggregated a-synuclein leads to transmissible spongiform encephalopathy. PLoS Pathog. 13(8): PMID: 28797122, PMCID:PMC5567908
Jarvela, T.S., Womack, T., Georgiou, P., Gould, T., Eriksen, J.L. and Lindberg, I. (2018) 7B2 chaperone knockout in APP model mice results in reduced plaque burden. Sci Rep. 8(1):9813. PMID: 29955078
Jarvela, T.S., Gahlot, S., Shakya, M., Bachor, T., White, A., Low, M.J., and Lindberg, I. (2019) Reduced stability and pH-dependent activity of a common obesity-linked PCSK1 polymorphism, N221D. Endocrinology 160(11):2630-2645. PMID: 31504391
Lindberg I. and Glembotski CC. (2019) Physiological control of signaling in the absence of amidated peptides. (Invited Commentary) Proc. Natl. Acad. Sci., 116(40):19774-19776. PMID: 31515450
Shakya, M., Yildirim, T., and Lindberg, I. (2020) Increased expression and retention of the secretory chaperone proSAAS following cell stress. Cell Stress and Chaperones 25(6), 929-941 PMID: 32607937
Jarvela, T.S., Chaplot, K., and Lindberg, I. (2021) A protease protection assay for the detection of internalized alpha-synuclein pre-formed fibrils. PlosOne 26;16(1):e0241161. doi: 10.1371/ journal.pone.0241161 PMID: 33497415
Shakya, M., Gahlot, S., White, A., Verchere. C.B., Low, M. J. and Lindberg, I. (2021) Mice lacking PC1/3 expression in POMC-expressing cells do not develop obesity. Endocrinology, 2021 Mar 10:bqab055. doi: 10.1210/endocr/bqab055. PMID: 33693631
Shakya, M.*, Gahlot, S. *, Martin, N.K., Arunagiri, A, Martin, M.G., Arvan, P., Low, M.J., and Lindberg, I. (2022) The G209R Pcsk1 mutant mouse as a model for human PCSK1 polyendocrinopathy. (*, co-first authors) Endocrinology Mar 4;163(5):bqac024. doi: 10.1210/endocr/bqac02 PMID: 35245347
Lindberg, I.*, Shu, Z.*, Lam, H.*, Helwig, M., Yucer, N., Laperle, A., Svendsen, C., Di Monte, D.A. and Maidment, N.T. (2022) The proSAAS chaperone provides neuroprotection and attenuates transsynaptic a-synuclein spread in rodent models of Parkinson's disease. (*, co-first authors) J. Parkinson’s Disease, May 4. doi: 10.3233/JPD-213053.. PMID: 3552756
Peinado, J.R.*, Chaplot, K.*, Jarvela, T.S., Barbieri, E.M, Shorter, J. and Lindberg, I. (2022) Sequestration of TDP-43(216-414) aggregates by cytoplasmic expression of the proSAAS chaperone. (*, co-first authors) ACS Chemical Neuroscience. Jun 1;13(11):1651-1665. doi: 10.1021/acschemneuro.2c00156. PMID: 35549000
Additional Publication Citations
Henrich, S. Lindberg, I., Bode, W., and Than, M.E. (2004) Proprotein convertase models based on the crystal structures of furin and kexin: explanation of their specificity. J. Biomol. Structure 345, 211-27.
Peinado, J.R.*, Laurent, V.*, Lee, S.N., Peng, B.W., Pintar, J.E., Steiner, D.F. and Lindberg, I. (2005) Strain-dependent influences on the hypothalamo-pituitary-adrenal axis profoundly affect the 7B2 null phenotype. (*co-first authors) Endocrinology 146, 3438-44.
Kacprzak, M.M., Than, M.E, Juliano, L., Juliano, M.A., Bode, W., and Lindberg, I. (2005) Mutagenesis of the PC2 substrate binding pocket alters enzyme specificity. J. Biol. Chem. 280, 31850-8.
Lindberg, I. (2005) Balancing risk and recovery. Science 310, 972.
Lee, S.N., Hwang, J.R., and Lindberg, I. (2006) The neuroendocrine protein 7B2 can be inactivated by phosphorylation within the secretory pathway. J. Biol. Chem. 281, 3312-20.
Ozawa A., Cai, Y., and Lindberg I. (2007) Production of bioactive peptides in an in vitro system. Analytical Biochem. 366:182-9.
Lee, S.N., Peng, B., Desjardins, R., Pintar, J.E., Day, R., and Lindberg, I. (2007) Strain-specific steroidal control of pituitary function. J. Endocrinol. 192: 515-25.
Lee, S.N. and Lindberg, I. (2008) 7B2 prevents unfolding and aggregation of prohormone convertase 2. Endocrinology 149: 4116-27.
Farber, C.R., Chitwood, J., Lee, S.N., Verdugo, R., Islas-Trejo, Rincon, A.G., Lindberg, I. and Medrano, J.F. (2008) Overexpression of Scg5 increases enzymatic activity of PCSK2 and is negatively correlated with weight gain in congenic mouse models. BMC Genet. 9:34 .
Kudo, H., Liu, J., Roubos, E., Ozawa, A., Panula, P. Martens, G.J.M., and Lindberg, I. (2009) Identification of proSAAS homologs in lower vertebrates: conservation of hydrophobic helices and convertase-inhibiting sequences. Endocrinology 150:1393-9.
Kowalska, D., Liu, J., Appel, J.R., Ozawa, A., Nefzi, A., Mackin, R.B., Houghten, R.A., and Lindberg, I. (2009) Synthetic small molecule prohormone convertase 2 inhibitors. Molecular Pharmacology 75: 617-25.
Ozawa, A., Peinado, J.R., and Lindberg, I. (2010) Modulation of prohormone convertase 1/3 properties using site-directed mutagenesis. Endocrinology 151: 4437-45.
Hoshino, A., Kowalska, D., Jean, F., Lazure, C., and Lindberg, I. (2011) Modulation of PC1/3 activity by self-interaction and substrate binding. Endocrinology, 152:1402-11
Helwig, M., Vivoli, M., Fricker, L.D., and Lindberg I. (2011) Regulation of neuropeptide processing enzymes by catecholamines in endocrine cells. Mol. Pharmacol. 80:304-13. PMID: 21540292
Helwig, M., Lee, S-N., Hwang, J.R., Ozawa, A., Medrano, J.F., and Lindberg, I. (2011) Dynamic modulation of PC2-mediated precursor processing by 7B2. J. Biol. Chem. 286:42504-13. PMID: 22013069
Vivoli, M., Caulfield, T.R., Martínez-Mayorga, K., Johnson, A.T., Jiao, G.-S. and Lindberg, I. (2012) Inhibition of PC1/3 and PC2 by 2,5-dideoxystreptamine derivatives. Mol. Pharmacol. 81(3):440-54. PMID: 22169851
Dasgupta, I.*, Sanglas, L.*, Enghild, J. and Lindberg I. (2012) The neuroendocrine protein 7B2 is an intrinsically disordered protein. (*co-first authors) Biochemistry 51(38):7456-64. PMID:22947085
Pickett, L.A., Yourshaw, M., Chen, Z. Solorzano-Vargas, R.S., Nelson, S.F., Martín, M.G., and Lindberg, I. (2013) Functional consequences of a novel variant of PCSK1. PLoS One 8(1):e55065. doi: 10.1371/journal.pone.0055065. PMID: 23383060; PMCID: PMC3557230
Helwig, M., Hoshino, A., Berridge, C., Lee, S.N., Lorenzen, N., Otzen, D., Eriksen, J., and Lindberg, I. (2013) The neuroendocrine protein 7B2 suppresses neurodegenerative disease-related protein aggregation. J. Biol. Chem. 288:1114–1124. PMID: 23172224
Martín, M.G., Lindberg, I, Solorzano-Vargas, R.S., Wang, J., Avitzur, Y., Bandsma, R., Sokollik, C., Lawrence, S., Pickett, L.A., Chen, Z., Egritas, O., Dalgic, B., Albornoz, V., de Ridder, L., Hulst, J., Gok, F., Aydoğan, A., Al-Hussaini, A., Gok, D.E., Yourshaw, M., Wu, S.V., Cortina, G., Stanford, S., and Georgia, S. (2013) Congenital proprotein convertase 1/3 deficiency causes malabsorptive diarrhea and other
Hoshino, A., Helwig, M., Rezaei, S., Berridge, C., Eriksen, J.L., and Lindberg, I. (2013) A novel function for proSAAS as an amyloid anti-aggregant in Alzheimer’s disease. J. Neurochem. 128(3):419-30. PMID: 24102330 PMCID: PMC3946950
Prabhu, Y., Blanco, E.H., Liu, M., Peinado, J.R., Wheeler, M., Gekakis, N., Arvan, P. and Lindberg, I. (2014) Defective transport of the obesity mutant PC1/3 N222D contributes to loss of function. Endocrinology 155(7):2391-401 PMID: 24828610
Blanco, E.H., Peinado, J.R, Martin, M.G., and Lindberg, I. (2014) Biochemical and cell biological properties of the human prohormone convertase 1/3 Ser357Gly mutation: a PC1/3 hypermorph. Endocrinology 55(9):3434-47 PMID: 24932808
Research Interests
Neurodegenerative Disease:
Alzheimer's disease is a devastating neurodegenerative disease presently affecting as many as 5.1 million Americans; the prevalence of the disease increases radically after age 65. Since the Census Bureau has estimated that the number of people over 65 will increase by 2050 to 88.5 million, Alzheimer’s disease presents a large future public health problem. The second most prevalent neurodegenerative disease is Parkinson’s disease, which presently affects over one million individuals in the United States.
Most neurodegenerative diseases involve defects in protein homeostasis that culminate in the production in excess quantities of cytotoxic aggregated proteins. Alzheimer’s disease involves the aberrant association of certain proteins, such as A-beta and tau, into insoluble formations in brain tissues known as plaques and tangles, while Parkinson’s disease involves the aggregation of a sticky protein known as alpha synuclein into Lewy bodies, the pathological hallmark of Parkinson’s. We have identified two secreted chaperone proteins, proSAAS and 7B2, synthesized predominantly in neural and endocrine cells, which we have shown can block the aggregation of sticky secretory molecules in cell line models.
We are especially interested in the role of these secretory chaperones in blocking the formation of protein aggregates in various neurodegenerative disease. Most recently we have shown that proSAAS can block the cytotoxic action of synuclein aggregates in a primary neuron model of Parkinson’s. This work involves immunohistochemistry (of brain tissue from Alzheimer’s and Parkinson’s patients); behavioral assays; cell culture models, and in vitro biochemistry
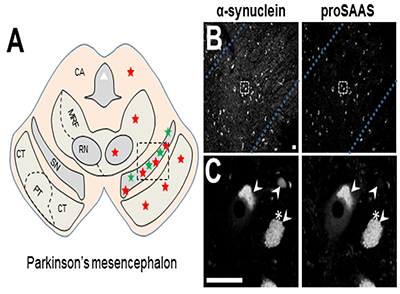
The neural chaperone proSAAS colocalizes with alpha synuclein in Lewy bodies.
Clinical Specialty Details
Awards and Affiliations
Professional Society Memberships
- 1978 ‐ present - Society for Neuroscience
- 1982 - present - Winter Conference on Brain Research
- 1985 ‐ present - American Society for Biochemistry and Molecular Biology
- 2001 ‐ present - The Endocrine Society
Career Development Awards
- 1981 - NIH Individual Postdoctoral Fellowship (switched to PRAT)
- 1981‐1983 - Pharmacology Research Associate Traineeship (PRAT)
- 1988‐1993 - Research Career Development Award (NIDDK)
- 1993‐1998 - Research Scientist Development Award (NIDA)
- 1998‐2003 - Research Scientist Development Award (NIDA)
Editorial Boards
- 2000-2005 - Editorial Board Member, Journal of Biological Chemistry
- 2012-2017 - Editorial Board Member, Journal of Biological Chemistry
Grants and Contracts
Ongoing Research Support
ProSAAS-mediated neuroprotective mechanisms in Alzheimer's and Parkinson's diseases: the role of secretory chaperones in neurodegeneration 2/01/19- 11/30/23
R01 AG062222-01 I. Lindberg and N. Maidment, Co-PIs (33% effort)
NIH/NIA
This grant is to investigate the ability of the neural secretory chaperone proSAAS to influence the deposition of amyloid into plaques, and synuclein into Lewy bodies.
Opioid Peptide Synthesizing Enzymes 7/01/17- 6/30/23
R01 DA042351-01A1 I. Lindberg (PI) (32% effort)
NIH/NIDA
This grant is to investigate the role of human PCSK1 mutations and polymorphisms in peptide-mediated hypothalamic obesity mechanisms.
Completed Research Support (Competing NIH applications and other grants)
1/85-12/86
I. Lindberg, PI
“Pharmacologic control of opioid peptide biosynthesis.”
Pharmaceutical Manufacturer’s Association Starter Grant
4/85-11/88
I. Lindberg, PI (30% effort)
“Biosynthesis of enkephalin in the adrenal medulla.”
R01 DK35199‐01
4/88‐3/91
I. Lindberg, PI (30% effort)
ʺOpioid peptide‐synthesizing enzymesʺ
R01 DA05084‐01
7/88-6/93
I. Lindberg, PI (90% salary)
Research Career Development Award
K04 DK 01868 (salary award)
12/88-11/91
I. Lindberg, PI(30% effort)
“Biosynthesis of enkephalin in the adrenal medulla.”
R01 DK 35199‐04
4/91-3/94
I. Lindberg, PI (30% effort)
ʺOpioid peptide‐synthesizing enzymesʺ
R01 DA 05084-04
10/93‐ 9/98
I. Lindberg, PI (75% salary)
Research Scientist Development Award
K02 DA 00204‐01 (salary award)
4/94-3/99
I. Lindberg, PI (30% effort)
ʺOpioid peptide‐synthesizing enzymesʺ
R01 DA 05084-07
7/96‐3/02
I. Lindberg, PI (30% effort)
“Control of peptide hormone biosynthesis by PC2 and 7B2”
R01 DK 49703-01
10/98-9/03
I. Lindberg, PI (75%)
Research Scientist Development Award
K02 DA 00204‐06 (salary award renewal)
4/99‐3/04
I. Lindberg, PI (30% effort)
“Opioid peptide‐synthesizing enzymesʺ
R01 DA 05084-12
4/02-3/07
I. Lindberg, PI (30% effort)
“Control of peptide hormone biosynthesis by PC2 and 7B2”
R01 DK 49703-06
2004
I. Lindberg, PI Gordon Conference support grant
“Proprotein processing, trafficking and secretion”
5R13 DK061936
2004
I. Lindberg, PI NSF Conference Support: received $2,000 for a poster award program for the same Gordon Conference cited above
4/04-3/09
I. Lindberg, PI (30% effort)
“Opioid peptide‐synthesizing enzymesʺ
R01 DA 05084-17
9/02‐8/05
I. Lindberg, PI (20% effort)
“Blockade of anthrax toxin cytotoxicity using furin inhibitors”
R21 AI 053517-01
8/03-8/06
P. Sunkara, PI (5% effort)
“Hexa-D-Arg: a furin inhibitor for anthrax biodefense”
Subcontract, Molecular Therapeutics
SBIR R43 AI 056850
9/04-3/06
S. Pincus, PI (5% effort)
“Furin Inhibition in HIV Disease”
R21 AI 058714-01
3/06‐6/06
I. Lindberg, PI (10% effort)
“Furin as an Anti-Cancer Target”
Louisiana Cancer Research Consortium
6/09- 5/11
I. Lindberg, PI (20% effort)
“Identification of Novel Peptide Hormones”
R21 DK084481-01
9/09- 3/14
I. Lindberg (P.I.) (30% effort)
“Control of peptide hormone biosynthesis by PC2 and 7B2”
R01 DK49703-12
7/09-6/14
I. Lindberg and B. Roth (co-P.I.s) (30% effort)
“De-orphanizing the peptidome”
R01 DA027170-01
Opioid Peptide Synthesizing Enzymes - 04/01/88-3/31/17
R56 DA05084-28A1 - I. Lindberg, PI
NIH/NIDA
This grant was to investigate the role of human PCSK1 mutations and polymorphisms in peptide-mediated obesity mechanisms.
The Secretory Chaperone 7B2 as an Endogenous Regulator of Amyloid Pathology - 09/01/14- 4/30/17
R21 AG045741-02 - I. Lindberg, PI
NIH/NIA
This grant was to test the hypothesis that brain 7B2 levels modulate the pathologic aggregation of beta amyloid-derived peptides.
Community Service
Treasurer, Greater Baltimore Society for Neuroscience, 2014-2019
Professional Activity
National Service
Journal Review
- 1990- present Ad hoc reviewer, J. Biol. Chem., J. Neurochem., Peptides, J. Neurosci., Analyt. Biochem., FEBS Lett., Protein Eng., Design and Selection, Proc. Natl Acad Sci, Endocrine Rev., Diabetes, Molecular Medicine and Metabolism, Mol. Cell. Endocrinol., J. Endocrinol., Endocrinol, PLoS ONE, Mol. Biol. Cell, Eur. J. Cell Biol., and others
- 2000-2005 Editorial Board Member,Journal of Biological Chemistry
- 2012-2017 Editorial Board Member, Journal of Biological Chemistry
Gordon Conference Service
- 1998, 2000 Advisory Committee Member, Gordon Conference: Hormonal and Neural Peptide Synthesis
- 2002 Vice‐Chair, Gordon Conference:Hormonal and Neural Peptide Synthesis
- 2004 Chair, Gordon Conference: Proprotein Processing, Trafficking, and Secretion
- 2006-2016 Advisory Committee Member, Gordon Conference:Proprotein Processing, Trafficking, and Secretion
Grant Reviewer, NIH:
- 1987 Study section reviewer, NLS1
- 1989, 1997 Study section reviewer, NIDA Biochemistry
- 1989, 1990 Special emphasis panel member, NIDDK
- 1991 Study section reviewer, NIMH career awards
- 1994 Special emphasis panel member, NINDS
- 1995 Study section reviewer, NLS1
- 1995, 1996, 1998 Study section and special emphasis panel reviewer, NIDDK
- 1996, 1997, 2002 Study section reviewer, NLS1
- 1996-2000 Standing member, Endocrinology study section
- 1999 Study section reviewer, ACS
- 2000 NIMH Career Awards study section reviewer
- 2000-2005 Endocrinology study section reviewer ( about 1 panel per year)
- 2007-2010 Standing member, Molecular and Cellular Endocrinology study section
- 2010 Special Emphasis Panel reviewer, NIDDK 2/2010
- 2011 EUREKA NIH review panel member
- 2013 SBIR study section, 3/2013
- 2015 MCE study section ad hoc service 6/2015; SBIR ad hoc service 10/2015
- 2016 SEP ad hoc service
- 2018 NIH internal review site visit 5/2018; NIDA internal review site visit, 12/2018
- 2020 NIH F05 Fellowship review panel 3/2020
[2022 R16 SURE award review panel ]
International Review Service
- 2002 Finnish National Academy of Sciences Review Panel, Helsinki, Finland
- 2002, 2009,2016 Canada Research Chairs, College of Reviewers
- 2003 Chair, Finnish National Academy of Sciences Review Panel
- 2005-present- many different Canadian and European granting agencies, including the Medical Research Council (U.K.), Wellcome Research Laboratories, Canadian Institutes of Health, the Danish Agency for Science Technology and Innovation, and the Belgian Foundation for Scientific Research
Lab Techniques and Equipment
Biochemistry and Molecular Biology: expression plasmid construction and mutagenesis; production of recombinant proteins, both in bacteria and in eukaryotic cell culture; protein purification and characterization; Western blotting; HPGPC and HPLC of proteins and peptides; radioimmunoassay and ELISA
Cell biology: Neuroendocrine and neural cell line management; metabolic labeling of cell lines, immunoprecipitation, phosphoimaging; confocal analysis of protein localization; protein turnover; cytotoxicity assays; lentiviral and AAV transduction
Animal model work: Alzheimer’s model mice; and 7B2 transgenic and knockout model mice. Mouse breeding and colony management; immunohistochemical analysis of brain tissue; viral injection
Major equipment: Akta Purifier, Shimadzu HPLC, Storm phosphoimager, Molecular Devices 96 - and 384-well fluorometer
